Development and Evaluation of Monoclonal Antibodies for Paxilline
Abstract
:1. Introduction
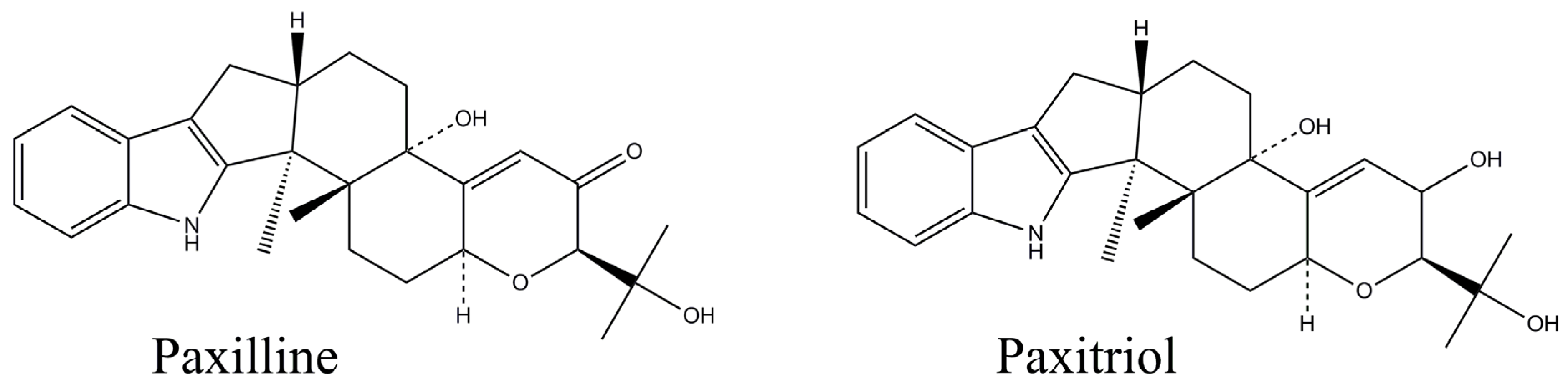
2. Results and Discussion
2.1. Production of mAbs to PAX

| Antibody | IC20 (ng/mL) | IC50 (ng/mL) | IC80 (ng/mL) | Dynamic Range (ng/mL) |
|---|---|---|---|---|
| 1-4 | 0.6 | 1.5 | 2.7 | 0.6–2.7 |
| 2-2 | 0.4 | 1.2 | 3.6 | 0.4–3.6 |
| 2-8 | 0.9 | 2.5 | 5.2 | 0.9–5.2 |
| 2-9 | 0.4 | 1.2 | 3.6 | 0.4–3.6 |
2.2. Effects of Solvents on mAb 2-9 ELISA
| PAX In: | Solvent Concentration a | IC50 (ng/mL) b | Relative Response (%) c | N d |
|---|---|---|---|---|
| PBS | 0% | 1.36 ± 0.16 | 100% | 20 |
| Methanol | 10% | 1.87 ± 0.21 | 72% | 6 |
| 20% | 3.03 ± 0.28 | 44% | 6 | |
| 30% | 4.03 ± 0.52 | 33% | 6 | |
| Acetonitrile | 5% | 3.72 ± 0.51 | 36% | 6 |
| 10% | 7.2 ± 1.1 | 18% | 6 | |
| 20% | 17.3 ± 3.3 | 7% | 6 |


2.3. Application of CI-ELISA to Silage Samples

| Spiking Level (µg/kg Dried Silage) | Recovery (% ±1 Std. Dev.) a |
|---|---|
| 100 | 106 ± 28 |
| 200 | 109 ± 18 |
| 500 | 106 ± 11 |
| 1000 | 102 ± 15 |
3. Experimental Section
3.1. Reagents
3.2. Ethical Statement
3.3. Development of PAX mAbs
3.4. Comparison of the PAX mAbs by CI-ELISA
3.5. Evaluation of Solvent Tolerance
3.6. Application to Silages
4. Conclusions
Acknowledgments
Disclaimer
Conflicts of Interest
References
- Sings, H.; Singh, S. Tremorgenic and nontremorgenic 2,3-fused indole diterpenoids. In The Alkaloids; Cordell, G.A., Ed.; Elsevier Academic Press: New York, NY, USA, 2003; pp. 51–163. [Google Scholar]
- Cole, R.J. Tremorgenic mycotoxins. In Mycotoxins in Human and Animal Health; Rodricks, J.V., Hesseltine, C.W., Mehlman, M.A., Eds.; Pathotox Publishers, Inc.: Park Forest South, IL, USA, 1977; pp. 583–595. [Google Scholar]
- Cole, R.J. Fungal tremorgens. J. Food Prot. 1981, 44, 715–722. [Google Scholar]
- Betina, V. Mycotoxins Chemical, Biological and Environmental Aspects; Elsevier Science Publishing Company, Inc.: New York, NY, USA, 1989; pp. 353–387. [Google Scholar]
- Nozawa, K. Tremorgenic mycotoxins having indoloditerpene moiety. Mycotoxins 1993, 37, 17–21. [Google Scholar] [CrossRef]
- Cole, R.J.; Kirksey, J.W.; Well, J.M. A new tremorgenic metabolite from Penicillium paxilli. Can. J. Microbiol. 1974, 20, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Knaus, H.G.; McManus, O.B.; Lee, S.H.; Schmalhofer, W.A.; Garcia-Calvo, M.; Helms, L.M.H.; Sanchez, M.; Giangiacomo, K.; Reuben, J.P. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 1994, 33, 5819–5828. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lingle, C.J. Paxilline inhibits BK channels by an almost exclusively closed-channel block mechanism. J. Gen. Physiol. 2014, 144, 415–440. [Google Scholar] [CrossRef] [PubMed]
- McLeay, L.M.; Smith, B.L.; Munday-Finch, S.C. Tremorgenic mycotoxins paxilline, penitrem and lolitrem B, the non-tremorgenic 31-epilolitrem B and electromyographic activity of the reticulum and rumen of sheep. Res. Vet. Sci. 1999, 66, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Imlach, W.L.; Finch, S.C.; Dunlop, J.; Meredith, A.L.; Aldrich, R.W.; Dalziel, J.E. The molecular mechanism of ryegrass staggers a neurological disorder of K+ channels. J. Pharmacol. Exp. Ther. 2008, 327, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J.; Dorner, J.W.; Lansden, J.A.; Cox, R.H.; Pape, C.; Cunfer, B.; Nicholson, S.S.; Bedell, D.M. Paspalum staggers: Isolation and identification of tremorgenic metabolites from sclerotia of Claviceps paspali. J. Agric. Food Chem. 1977, 25, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Sabater-Vilar, M.; Nijmeijer, S.; Fink-Gremmels, J. Genotoxicity assessment of five tremorgenic mycotoxins (fumitremorgen B, paxilline, penitrem A, verruculogen, and verrucosidin) produced by molds isolated from fermented meats. J. Food Prot. 2003, 66, 2123–2129. [Google Scholar] [PubMed]
- Gürbüzel, M.; Uysal, H.; Kizilet, H. Assessment of genotoxicity of the mycotoxin paxilline using the somatic mutation and recombination test in Drosophila melanogaster. Fresenius Environ. Bull. 2010, 19, 1038–1041. [Google Scholar]
- Andersen, B.; Frisvad, J.C. Natural occurrence of fungi and fungal metabolites in moldy tomatoes. J. Agric. Food Chem. 2004, 52, 7507–7513. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.K. Analysis of endophyte toxins: Fescue and other grasses toxic to livestock. J. Anim. Sci. 1995, 73, 871–880. [Google Scholar] [PubMed]
- Garthwaite, I.; Miles, C.O.; Towers, N.R. Immunological detection of the indole diterpenoid tremorgenic mycotoxins. In Proceedings of the Second International Symposium on Acremonium/Grass Interactions; Hume, D.E., Latch, G.C.M., Easton, H.S., Eds.; AgResearch, Grasslands Research Centre: Palmerston North, New Zealand, 1993; pp. 77–80. [Google Scholar]
- Fueki, S.; Tokiwano, T.; Toshima, H.; Oikawa, H. Biosynthesis of indole diterpenes, emindole, and paxilline: Involvement of a common intermediate. Org. Lett. 2004, 6, 2697–2700. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Noike, M.; Minami, A.; Oikawa, H.; Dairi, T. Functional analysis of a prenyltransferase gene (paxD) in the paxilline biosynthetic gene cluster. Appl. Microbiol. Biotechnol. 2014, 98, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Selala, M.I.; Musuku, A.; Schepens, P.J.C. Isolation and determination of paspalitrem-type tremorgenic mycotoxins using liquid chromatography with diode-array detection. Anal. Chim. Acta 1991, 244, 1–8. [Google Scholar] [CrossRef]
- Young, C.; Itoh, Y.; Johnson, R.; Garthwaite, I.; Miles, C.O.; Munday-Finch, S.C.; Scott, B. Paxilline-negative mutants of Penicillium paxilli generated by heterologous and homologous plasmid integration. Curr. Genetics 1998, 33, 368–377. [Google Scholar] [CrossRef]
- Maragos, C. Photoreaction of indole-containing mycotoxins to fluorescent products. Mycotoxin Res. 2009, 25, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.O.; Grewal, P.S. Does decreased mowing frequency enhance alkaloid production in endophytic tall fescue and perennial ryegrass? J. Chem. Ecol. 2002, 28, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Egge-Jacobsen, W.; Vrålstad, T.; Miles, C.O. Indole-diterpenoid profiles of Claviceps paspali and Claviceps purpurea from high-resolution Fourier transform Orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, V.; Sulyok, M.; Labuda, R.; Bicker, W.; Krska, R. Simultaneous determination of 186 fungal and bacterial metabolites in indoor matrices by liquid chromatography/tandem mass spectrometry. Anal. Bioanal. Chem. 2009, 395, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, I.; Miles, C.O.; Towers, N.R. An immunological approach to the study of the ryegrass staggers syndrome. Bull. OILB/SROP 1994, 17, 175–180. [Google Scholar]
- Miles, C.O.; Wilkins, A.L.; Garthwaite, I.; Ede, R.M.; Munday-Finch, S.C. Immunochemical techniques in natural products chemistry: Isolation and structure determination of a novel indole-diterpenoid aided by TLC-ELISAgram. J. Org. Chem. 1995, 60, 6067–6069. [Google Scholar] [CrossRef]
- Randox Food Diagnostics. Available online: http://www.randoxfooddiagnostics.com/mycotoxin-array (accessed on 29 July 2015).
- Kim, E.K.; Maragos, C.M.; Kendra, D.F. Liquid chromatographic determination of fumonisins B1, B2, and B3 in corn silage. J. Agric. Food Chem. 2004, 52, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Wilkins, A.L.; Gallagher, R.T.; Hawkes, A.D.; Munday, S.C.; Towers, N.R. Synthesis and tremorgenicity of paxitriols and lolitriol: Possible biosynthetic precursors of lolitrem B. J. Agric. Food Chem. 1992, 40, 234–238. [Google Scholar] [CrossRef]
- Lau, H.P.; Gaur, P.K.; Chu, F.S. Preparation and characterization of aflatoxin B2a-hemiglutarate and its use for the production of antibody against aflatoxin B1. J. Food Saf. 1981, 3, 1–13. [Google Scholar] [CrossRef]
- Chu, F.S.; Hsia, S.; Sun, P.S. Preparation and characterization of aflatoxin B1-1-(O-carboxymethyl) oxime. J. AOAC 1977, 60, 791–794. [Google Scholar]
- Maragos, C.M.; Kurtzman, C.; Busman, M.; Price, N.; McCormick, S. Development and evaluation of monoclonal antibodies for the glucoside of T-2 toxin (T2-Glc). Toxins 2013, 5, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Hoogenraad, N.; Newman, T.; Hoogenraad, J. The effect of pre-injection of mice with pristane on ascites tumour formation and monoclonal antibody production. J. Immunol. Methods 1983, 61, 317–320. [Google Scholar] [CrossRef]
- Maragos, C.M.; McCormick, S.P. Monoclonal antibodies for the mycotoxins deoxynivalenol and 3-acetyl-deoxynivalenol. Food Agric. Immunol. 2000, 12, 181–192. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maragos, C.M. Development and Evaluation of Monoclonal Antibodies for Paxilline. Toxins 2015, 7, 3903-3915. https://doi.org/10.3390/toxins7103903
Maragos CM. Development and Evaluation of Monoclonal Antibodies for Paxilline. Toxins. 2015; 7(10):3903-3915. https://doi.org/10.3390/toxins7103903
Chicago/Turabian StyleMaragos, Chris M. 2015. "Development and Evaluation of Monoclonal Antibodies for Paxilline" Toxins 7, no. 10: 3903-3915. https://doi.org/10.3390/toxins7103903