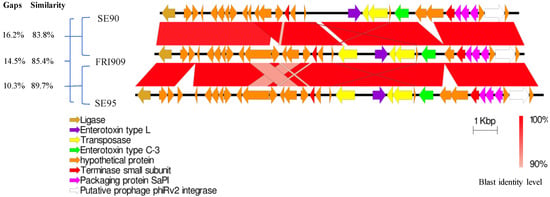
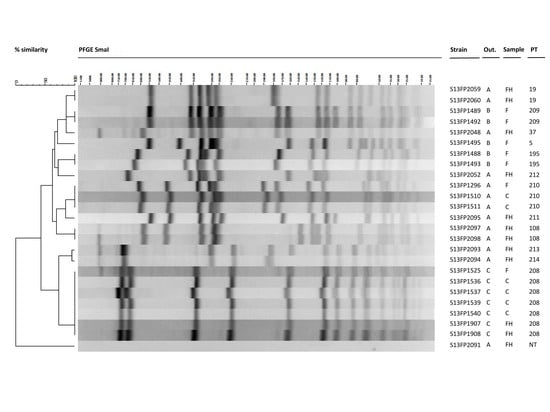
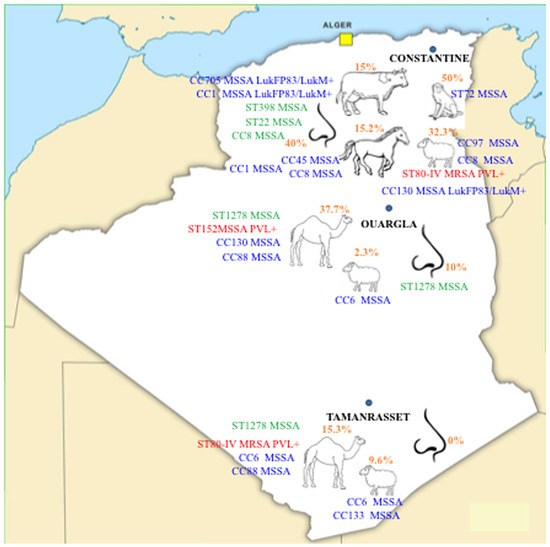
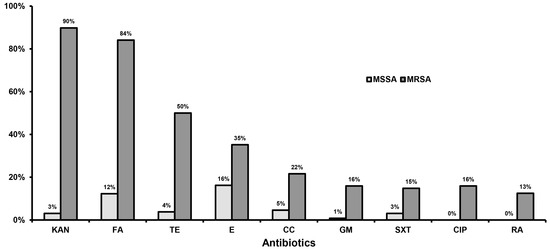
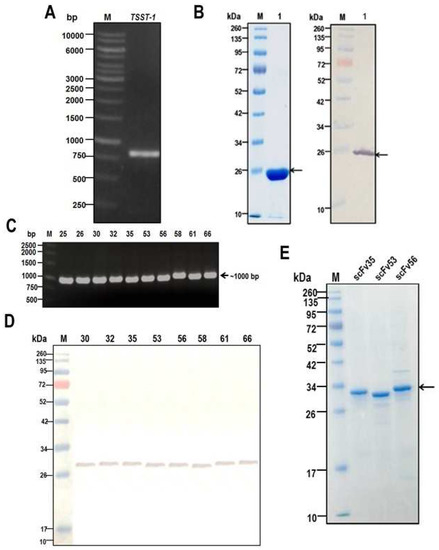
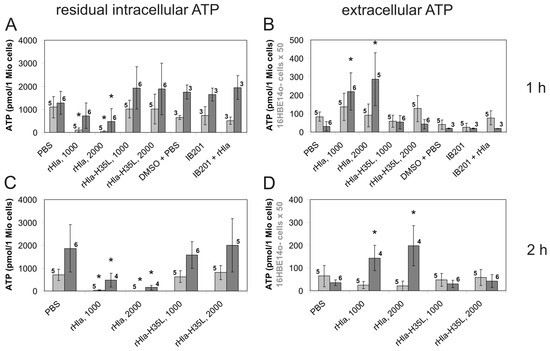
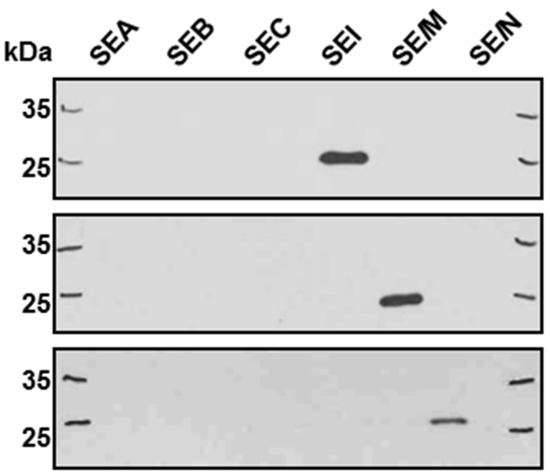
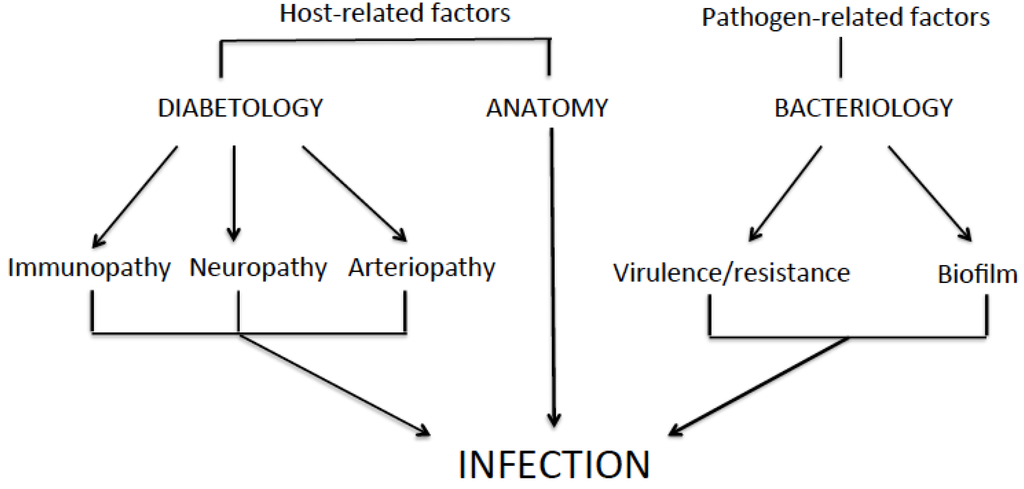
Staphylococcus aureus Toxins
A topical collection in Toxins (ISSN 2072-6651). This collection belongs to the section "Bacterial Toxins".
Viewed by 289644Editor
Interests: Staphylococcus aureus; MRSA; two-component regulatory systems; toxins; essential proteins; gene regulation; antibacterial drug discovery; host pathogen interactions
Topical Collection Information
Dear Colleagues,
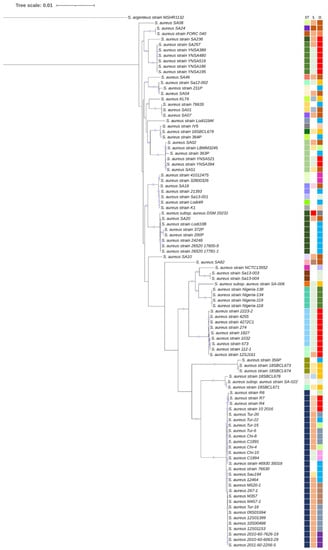
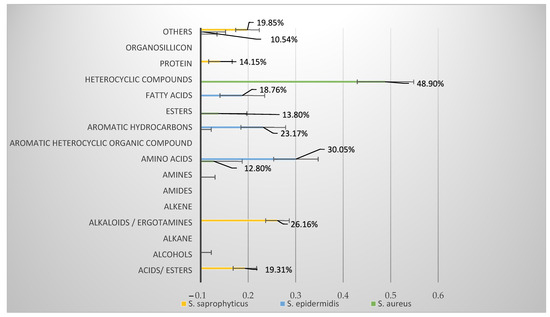
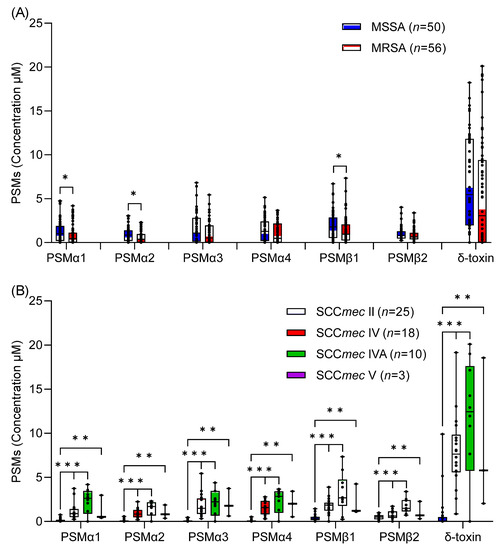
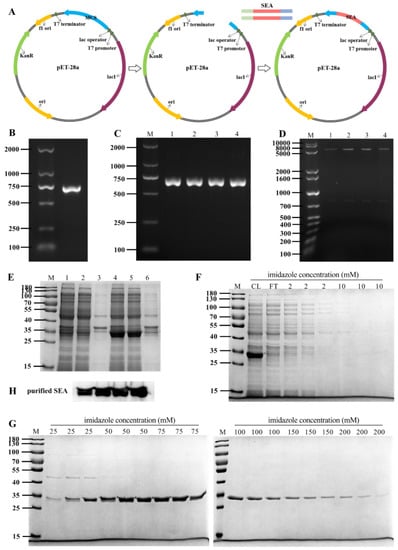
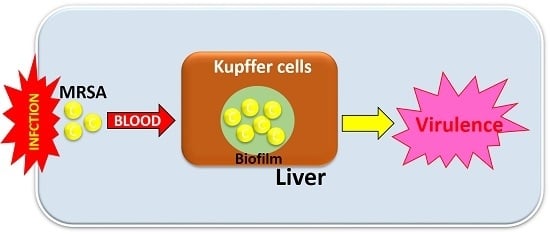
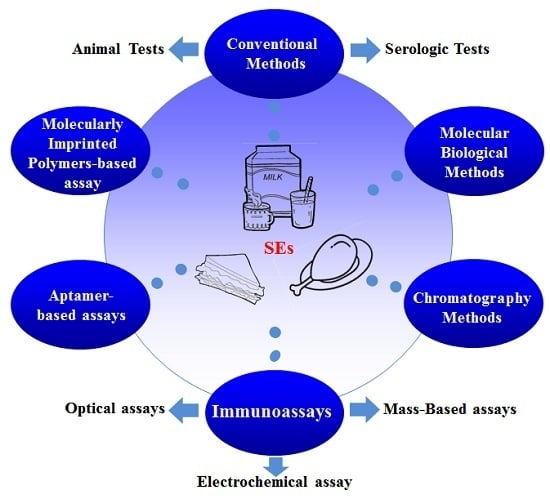
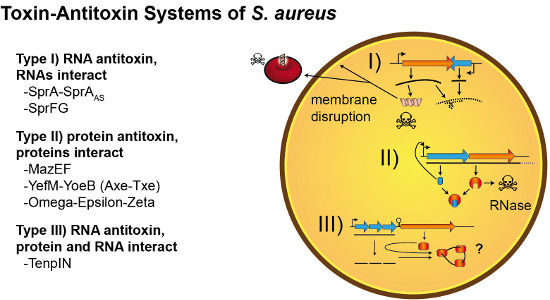
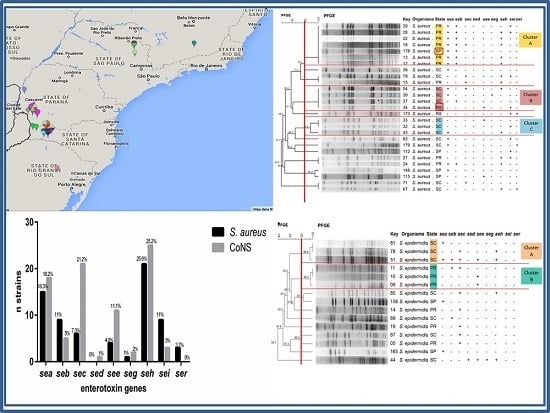
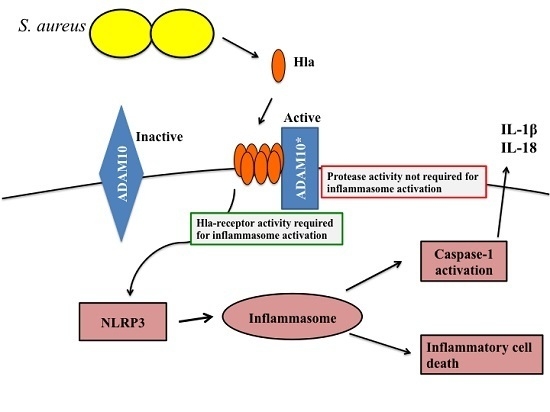
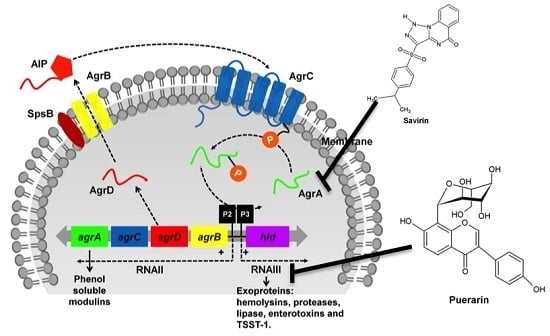
Staphylococcus aureus is a major pathogen that causes a variety of infections, including commonly superficial skin and soft tissue infections and severe systematic infections. The ability of this organism to cause significant illness is mainly due to its production of numerous toxins, such as α-, β-, γ-, and δ-toxins, Panton-Valentine leukocidin, Phenol soluble modulins (PSM), enterotoxins, and toxic shock syndrome toxin-1. It has been revealed that these toxins are able to bind to distinct receptors and trigger various signaling pathways and lead to inflammatory responses and cell death during host-cell pathogen interactions. This Special Issue of Toxins will cover reviews and research articles on the advancements in the field of cellular and molecular pathogenesis of staphylococcal toxins, toxoid vaccine development, and regulatory mechanism of toxin production. Animal models used for validation the pathogenicity of staphylococcal toxins will also be covered in this Special Issue.
Prof. Dr. Yinduo Ji
Collection Editor
Submission
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission formAll papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are refereed through a peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Toxins is an international peer-reviewed Open Access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1200 CHF (Swiss Francs).
Keywords
- toxin
- hemolycin
- leukotoxin
- enterotoxin
- superantigen
- signaling pathway
- cytotoxicity
- gene regulation
- toxoid
- vaccine