Antibiotics 2022, 11(6), 784; https://doi.org/10.3390/antibiotics11060784 - 8 Jun 2022
Cited by 95 | Viewed by 12076
Abstract
Artificial intelligence (AI) is a branch of science and engineering that focuses on the computational understanding of intelligent behavior. Many human professions, including clinical diagnosis and prognosis, are greatly useful from AI. Antimicrobial resistance (AMR) is among the most critical challenges facing Pakistan
[...] Read more.
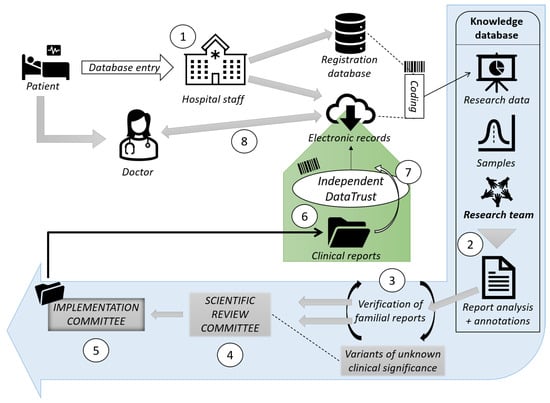
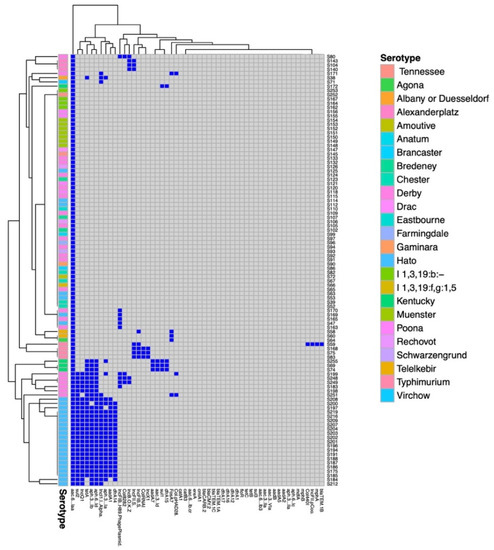
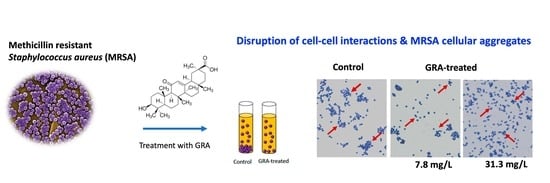
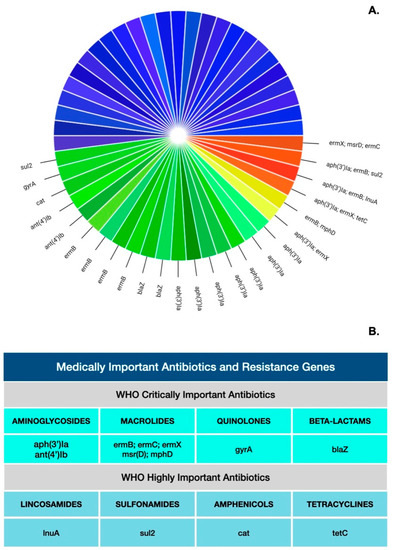
Artificial intelligence (AI) is a branch of science and engineering that focuses on the computational understanding of intelligent behavior. Many human professions, including clinical diagnosis and prognosis, are greatly useful from AI. Antimicrobial resistance (AMR) is among the most critical challenges facing Pakistan and the rest of the world. The rising incidence of AMR has become a significant issue, and authorities must take measures to combat the overuse and incorrect use of antibiotics in order to combat rising resistance rates. The widespread use of antibiotics in clinical practice has not only resulted in drug resistance but has also increased the threat of super-resistant bacteria emergence. As AMR rises, clinicians find it more difficult to treat many bacterial infections in a timely manner, and therapy becomes prohibitively costly for patients. To combat the rise in AMR rates, it is critical to implement an institutional antibiotic stewardship program that monitors correct antibiotic use, controls antibiotics, and generates antibiograms. Furthermore, these types of tools may aid in the treatment of patients in the event of a medical emergency in which a physician is unable to wait for bacterial culture results. AI’s applications in healthcare might be unlimited, reducing the time it takes to discover new antimicrobial drugs, improving diagnostic and treatment accuracy, and lowering expenses at the same time. The majority of suggested AI solutions for AMR are meant to supplement rather than replace a doctor’s prescription or opinion, but rather to serve as a valuable tool for making their work easier. When it comes to infectious diseases, AI has the potential to be a game-changer in the battle against antibiotic resistance. Finally, when selecting antibiotic therapy for infections, data from local antibiotic stewardship programs are critical to ensuring that these bacteria are treated quickly and effectively. Furthermore, organizations such as the World Health Organization (WHO) have underlined the necessity of selecting the appropriate antibiotic and treating for the shortest time feasible to minimize the spread of resistant and invasive resistant bacterial strains.
Full article
(This article belongs to the Special Issue Artificial Intelligence and Machine Learning Techniques for Epidemiology, Diagnostic and Treatment of Infectious Diseases)
►
Show Figures