Cancers 2020, 12(10), 3031; https://doi.org/10.3390/cancers12103031 - 18 Oct 2020
Cited by 5 | Viewed by 3165
Abstract
While the emotional response of healthcare providers during the COVID-19 pandemic has been extensively investigated in countries in the Far-East, little is known about the psychological impact and the associated emotional distress of healthcare providers in Italy, especially with regard to different regions.
[...] Read more.
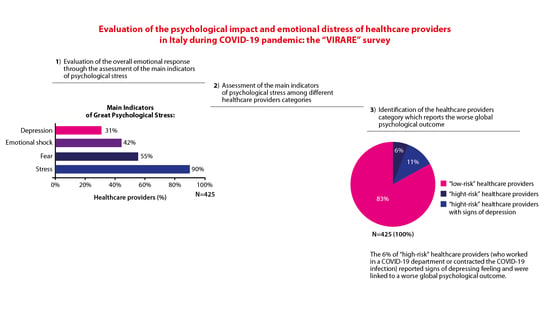
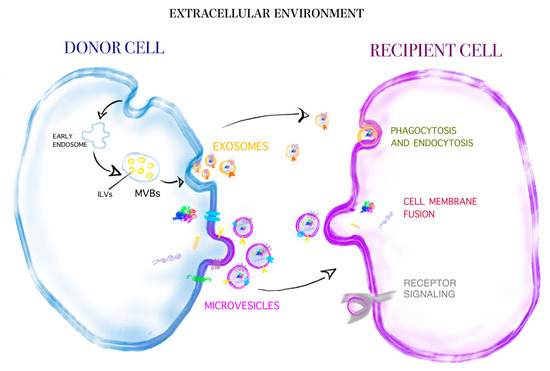
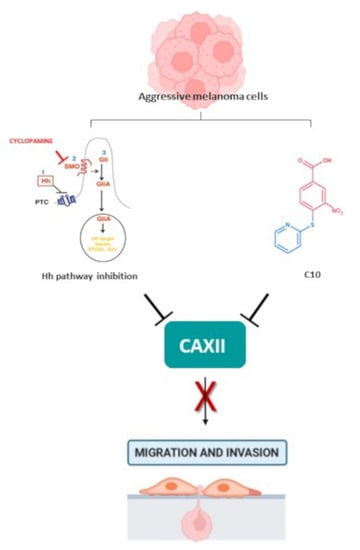
While the emotional response of healthcare providers during the COVID-19 pandemic has been extensively investigated in countries in the Far-East, little is known about the psychological impact and the associated emotional distress of healthcare providers in Italy, especially with regard to different regions. The aim of the “VIRARE” survey, which was addressed to all the healthcare providers in the Lazio region (central Italy) and, in particular, to those working in the oncology field, is to analyze their opinion on the impact and management of the pandemic, to better understand the level of their psychological distress. A global good psychological response of healthcare providers to the pandemic has emerged, independently from their different occupations in the oncology field. Healthcare providers show a high degree of resilience, identifying the major causes of distress the difficulty of the management of this situation, the obstacles in their working activity and expressing a high degree of dissatisfaction with how Italian institutions handled this situation. This survey also provides a direct comparison between COVID-19-infected (or directly in contact with COVID-19-infected patients) and uninfected healthcare providers, identifying the sub-category of infected professionals that reported signs of depression as particularly vulnerable.
Full article
(This article belongs to the Collection The Impact of COVID-19 Infection in Cancer)
►
Show Figures