Coating Deposition and Surface Functionalization of Implants for Biomedical Applications
A topical collection in Journal of Functional Biomaterials (ISSN 2079-4983).
Viewed by 44199Editor
Interests: composite materials; functional materials; cementitious composites; additive manufacturing
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
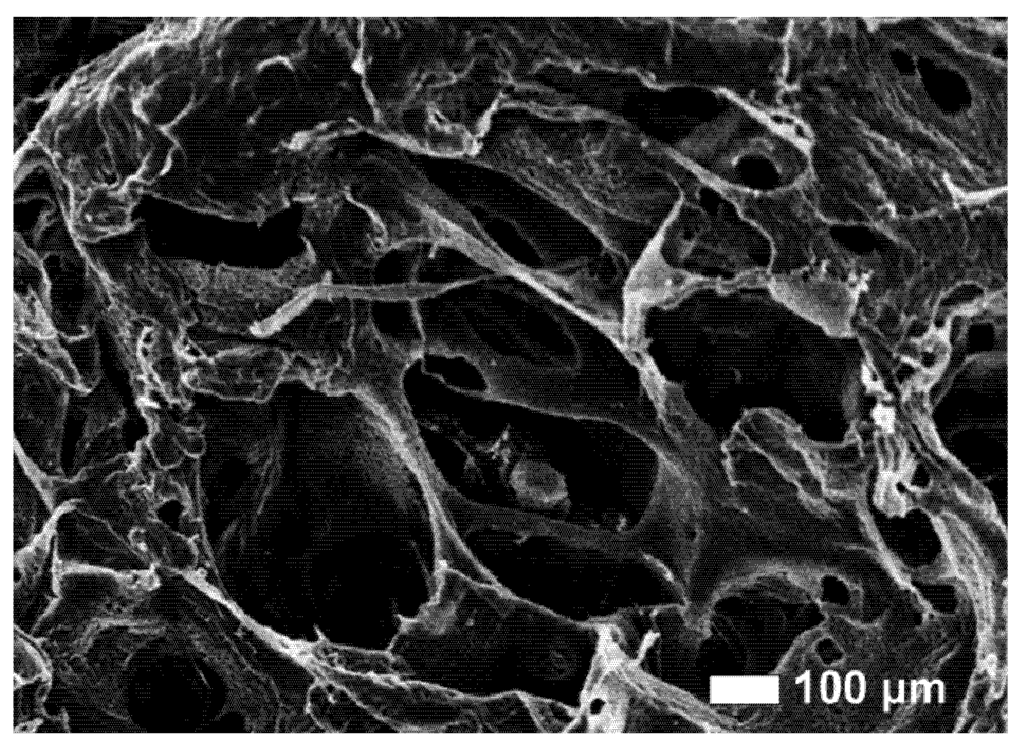
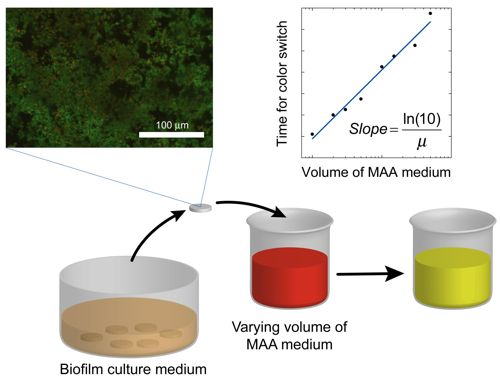
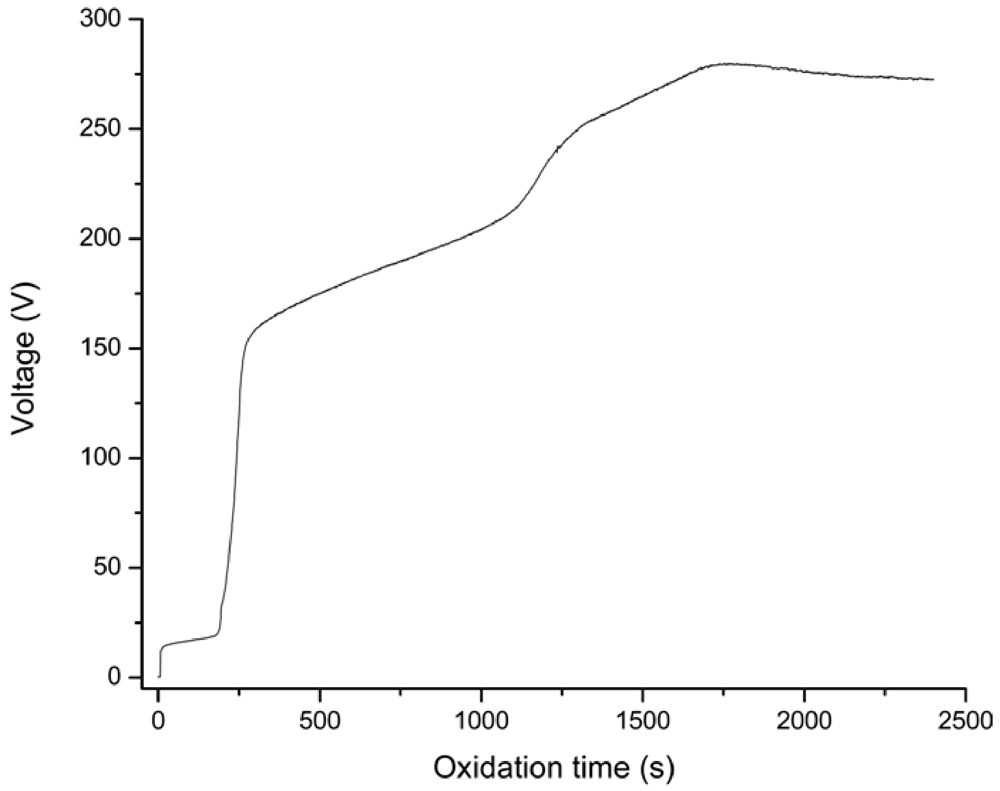
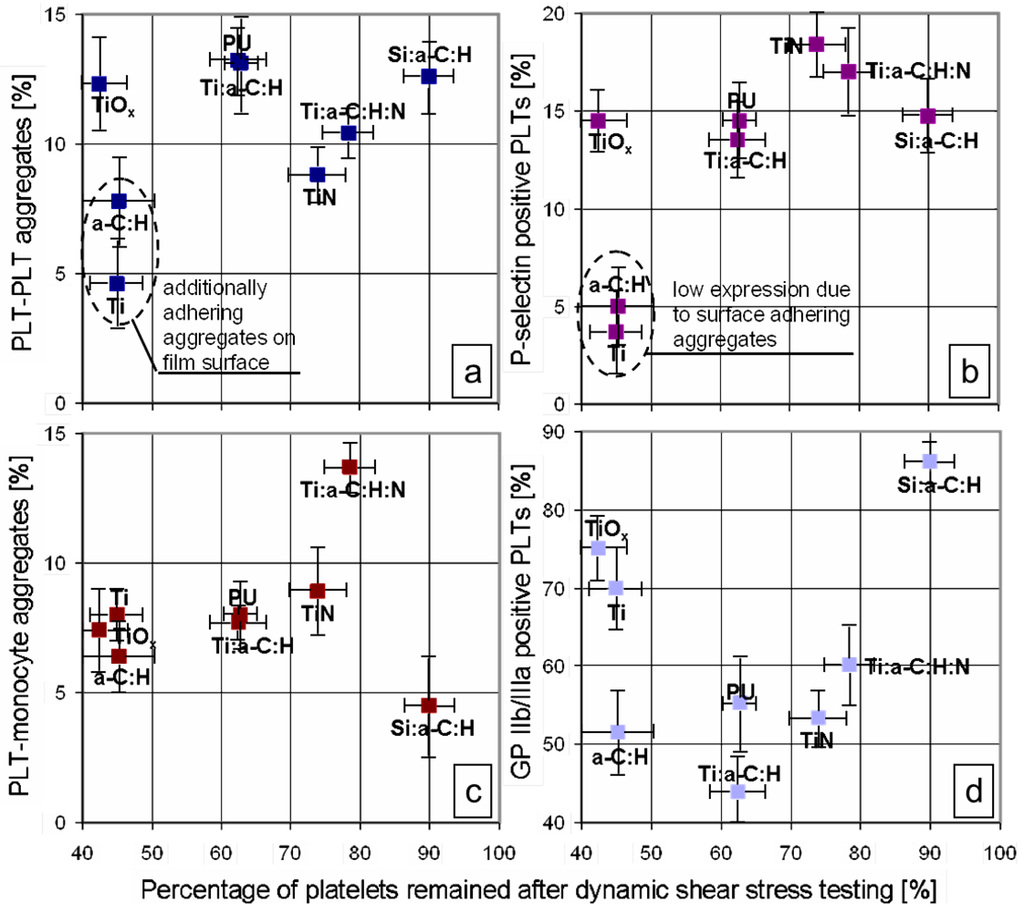
The human body is able to promote spontaneous healing phenomena to face the adverse consequences of diseases, aging processes or traumatic events. However such natural reactions are not always sufficient to recover extensive functional losses and, in that case, medical or surgical interventions are required. For this reason, the demand for new biomaterials to support or restore the role of damaged tissues is a major clinical and socioeconomic need. The deposition of a proper coating or the chemicophysical treatment of the surface may boost the performance of implant devices, conveying site-specific properties to the substrate material. The apposition of glass-based glazes which resemble the original enamel of tooth, the chemical modification of titanium to activate the bone-bonding ability or the deposition of calcium-phosphate layers on metal substrates to elicit the surface development of hydroxyapatite are just a few examples of the new approaches to improve the behaviour of medical grafts by means of biocoatings and surface functionalization methods.
Dr. Antonella Sola
Collection Editor
Manuscript Submission Information
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Please visit the Instructions for Authors page before submitting a manuscript. The article processing charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). English correction and/or formatting fees will be charged in certain cases for those articles accepted for publication that require extensive additional formatting and/or English corrections. For further details see here.
Keywords
- biocoatings
- functionalization
- surface treatments
- coating deposition methods
- implant materials