1
Sorbonne Paris Nord University, Chemistry Structures Properties of Biomaterials and Therapeutic Agents Laboratory (CSPBAT), Nanomédecine Biomarqueurs Détection Team (NBD), The National Center for Scientific Research (CNRS), UMR 7244, 74 Rue Marcel Cachin, CEDEX, 93017 Bobigny, France
2
CAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
3
School of Pharmacy, Anhui Medical University, Hefei 230032, China
4
Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA
5
Université d’Evry Val d’Essonne—Université Paris-Saclay, 91000 Evry, France
6
Muséum National d’Histoire Naturelle, Unité MCAM, UMR 7245, CNRS, 75005 Paris, France
7
Department of Pathology, University Hospital Jean Verdier, Assistance Publique-Hôpitaux de Paris, 93140 Paris, France
8
Department of Digestive and Metabolic Surgery, Jean Verdier Hospital, Paris XIII University—University Hospitals of Paris Seine Saint-Denis, 93140 Paris, France
9
Université Paris Cité, CNRS, INSERM, Institut Cochin, 75014 Paris, France
†
These authors contributed equally to this work.
Metabolites 2022, 12(11), 1081; https://doi.org/10.3390/metabo12111081 - 8 Nov 2022
Cited by 5 | Viewed by 4656
Abstract
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a complex disorder that is implicated in dysregulations in multiple biological pathways, orchestrated by interactions between genetic predisposition, metabolic syndromes and environmental factors. The limited knowledge of its pathogenesis is one of the bottlenecks in the
[...] Read more.
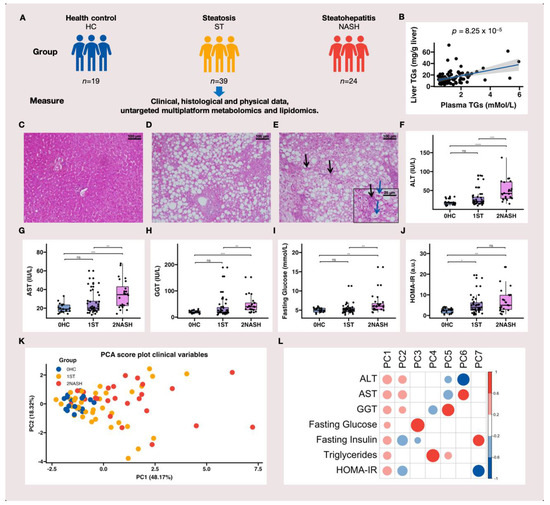
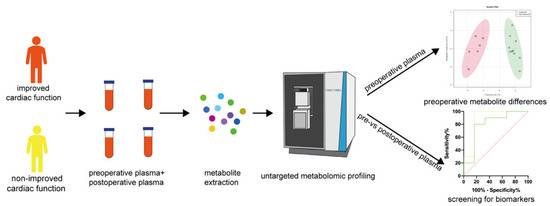
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a complex disorder that is implicated in dysregulations in multiple biological pathways, orchestrated by interactions between genetic predisposition, metabolic syndromes and environmental factors. The limited knowledge of its pathogenesis is one of the bottlenecks in the development of prognostic and therapeutic options for MAFLD. Moreover, the extent to which metabolic pathways are altered due to ongoing hepatic steatosis, inflammation and fibrosis and subsequent liver damage remains unclear. To uncover potential MAFLD pathogenesis in humans, we employed an untargeted nuclear magnetic resonance (NMR) spectroscopy- and high-resolution mass spectrometry (HRMS)-based multiplatform approach combined with a computational multiblock omics framework to characterize the plasma metabolomes and lipidomes of obese patients without (n = 19) or with liver biopsy confirmed MAFLD (n = 63). Metabolite features associated with MAFLD were identified using a metabolome-wide association study pipeline that tested for the relationships between feature responses and MAFLD. A metabolic pathway enrichment analysis revealed 16 pathways associated with MAFLD and highlighted pathway changes, including amino acid metabolism, bile acid metabolism, carnitine shuttle, fatty acid metabolism, glycerophospholipid metabolism, arachidonic acid metabolism and steroid metabolism. These results suggested that there were alterations in energy metabolism, specifically amino acid and lipid metabolism, and pointed to the pathways being implicated in alerted liver function, mitochondrial dysfunctions and immune system disorders, which have previously been linked to MAFLD in human and animal studies. Together, this study revealed specific metabolic alterations associated with MAFLD and supported the idea that MAFLD is fundamentally a metabolism-related disorder, thereby providing new perspectives for diagnostic and therapeutic strategies.
Full article
(This article belongs to the Special Issue Biofluid-Based Metabolomics for Biomarker Discovery)
▼
Show Figures