Cancers 2020, 12(9), 2648; https://doi.org/10.3390/cancers12092648 - 16 Sep 2020
Cited by 18 | Viewed by 3091
Abstract
Pathological complete response (pCR) after neoadjuvant chemotherapy (NACT) can predict better survival outcomes in patients with early triple negative breast cancer (TNBC). Tumor infiltrating lymphocytes (TILs), Programmed Death-Ligand 1 (PD-L1), and Cluster of Differentiation 73 (CD73) are immune-related biomarkers that can be evaluated
[...] Read more.
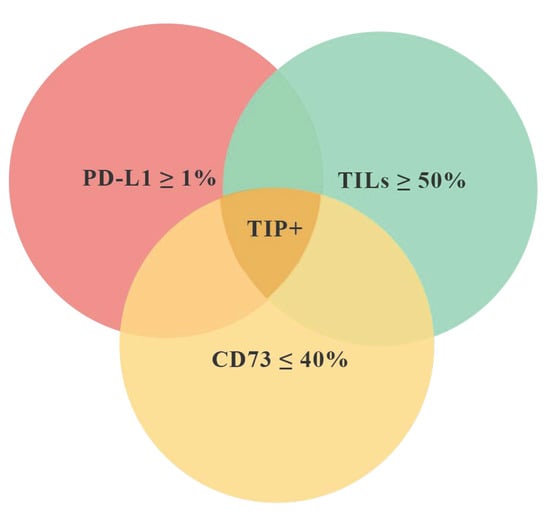
Pathological complete response (pCR) after neoadjuvant chemotherapy (NACT) can predict better survival outcomes in patients with early triple negative breast cancer (TNBC). Tumor infiltrating lymphocytes (TILs), Programmed Death-Ligand 1 (PD-L1), and Cluster of Differentiation 73 (CD73) are immune-related biomarkers that can be evaluated in the tumor microenvironment. We investigated if the contemporary expression of these biomarkers combined in a tissue immune profile (TIP) can predict pCR better than single biomarkers in TNBC. Tumor infiltrating lymphocytes (TILs), CD73 expression by cancer cells (CC), and PD-L1 expression by immune cells (IC) were evaluated on pre-NACT biopsies. We defined TIP positive (TIP+) as the simultaneous presence of TILS ≥ 50%, PD-L1 ≥ 1%, and CD73 ≤ 40%. To consider the effects of all significant variables on the pCR, multivariate analysis was performed. Akaike information criterion (AIC) and Bayesian information criterion (BIC) were used for model selection. We retrospectively analyzed 60 biopsies from patients with TNBC who received standard NACT. Pathological complete response was achieved in 23 patients (38.0%). Twelve (20.0%) cases resulted to be TIP+. The pCR rate was significantly different between TIP+ (91.7%) and TIP− (25.0%) (p < 0.0001). Using a multivariate analysis, TIP was confirmed as an independent predictive factor of pCR (OR 49.7 (6.30–392.4), p < 0.0001). Finally, we compared the efficacy of TIP versus each single biomarker in predicting pCR by AIC and BIC. The combined immune profile is more accurate in predicting pCR (AIC 68.3; BIC 74.5) as compared to single biomarkers. The association between TIP+ and pCR can be proposed as a novel link between immune background and response to chemotherapy in TNBC, highlighting the need to consider an immunological patients’ profile rather than single biomarkers.
Full article
(This article belongs to the Special Issue Neoadjuvant Systemic Therapy in Early Breast Cancer)
►
Show Figures