Assessment of Information to Substantiate a Health Claim on the Prevention of Prostate Cancer by Lignans
Abstract
:1. Introduction
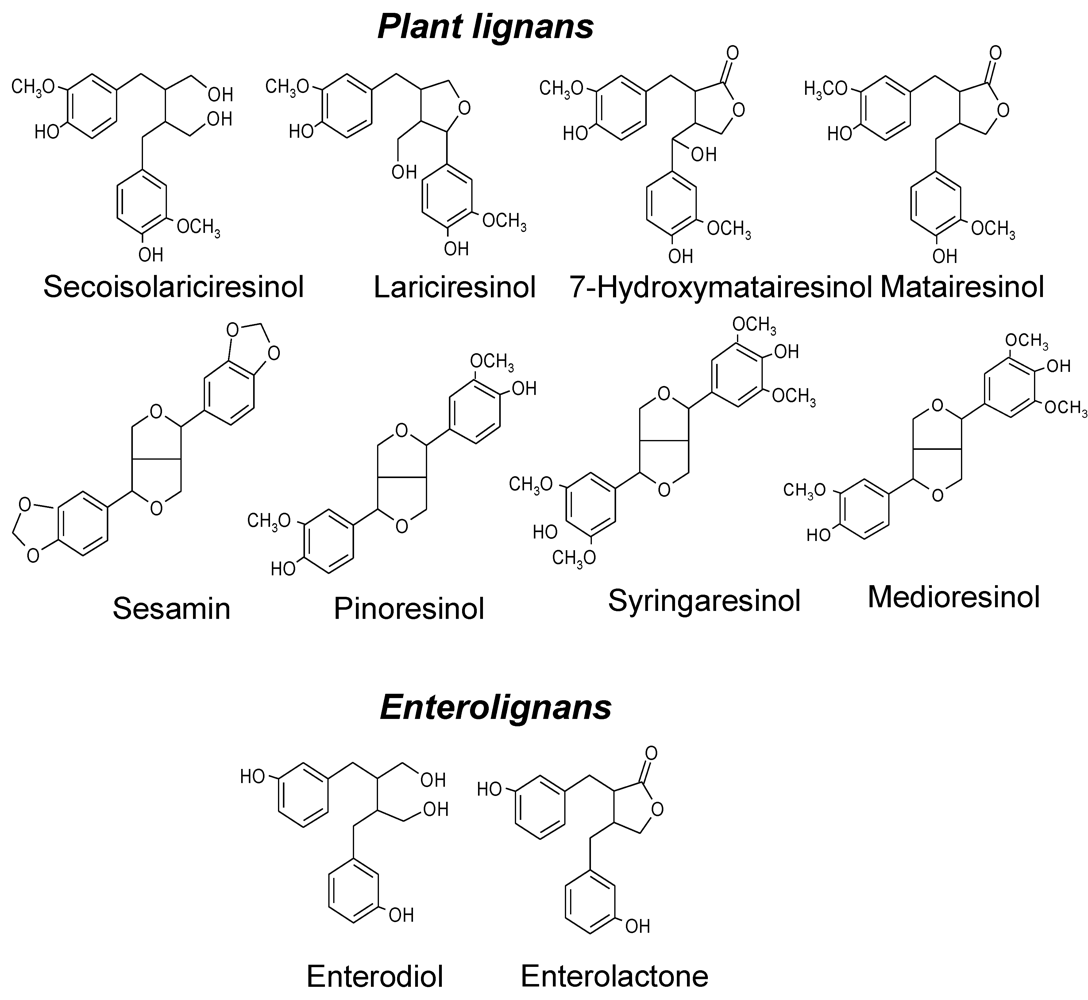
2. Epidemiological Studies on Lignans and Prostate Cancer Risk
| Type of Study | Study population | Outcome | Ref. |
|---|---|---|---|
| Nested Case-Control | Multiethnic cohort in Hawaii and Califormia, 249 cases, 404 controls | No association between urinary ENL excretion and prostate cancer risk. | [68] |
| Nested Case-Control | EPIC cohort, 950 cases, 1042 controls | No association between plasma enterodiol or ENL and prostate cancer risk. | [13] |
| Nested Case-Control | EPIC-Norfolk cohort, 193 cases, 828 controls | No association between prostate cancer risk and total serum lignans or enterodiol or ENL. | [20] |
| Prospective | British men, 191 prostate cancer patients, 71 progressed diseases during median of 2.5 years follow-up | No association of baseline urinary ENL levels with time to disease progression. No association between adverse histology on repeat biopsy or PSA velocity and urinary ENL. | [69] |
| Case-Control | Scottish men, 50-74 y, 433 cases, 483 controls | Inverse association with increased serum concentrations of ENL and prostate cancer risk. | [19] |
| Case-Control | Swedish men, 35-79 y, 1499 cases, 1130 controls; 209 cases, 214 controls for assessment of serum enterolactone | No association between dietary intake of total or individual lignans or isoflavonoids and risk of prostate cancer. Intermediate serum levels of ENL were associated with a decreased risk of prostate cancer. | [15] |
| Case-Control | Men in Western New York, 433 cases, 538 controls | Reduced risk of prostate cancer in men in the highest quartile of intake of total lignan precursors* compared with men in the lowest quartile of intake. | [2] |
| Prospective | Swedish men, 265 cases, 525 controls. Mean follow-up 5 years | No significant association between quartiles of plasma ENL and risk of prostate cancer. | [18] |
| Nested Case-Control | Finnish male smokers, 50-69 y, 214 cases, 214 controls | No association between serum ENL concentrations and prostate cancer risk. | [17] |
| Case-Control | Caucasian men in Texas, 83 cases, 107 controls | No association between high intake of lignans** and prostate cancer risk. | [14] |
| Nested Case-Control | Finnish, Norwegian and Swedish men, 794 cases, 2550 controls | No association between serum ENL concentrations and prostate cancer risk in full study group or in national groups. | [16] |
3. Preclinical in vivo Studies of Lignans and Lignan-Rich Foods
4. Potential Mechanisms of Action of Lignans in Prostate Cancer
5. Surrogate Endpoints for Clinical Interventions
6. Clinical Intervention Studies
| Study subjects | Intervention | n | Duration | Effects of intervention | Ref. |
|---|---|---|---|---|---|
| Men with PIN scheduled repeated biopsies | Flaxseed 30 g/day combined with low-fat (≤20% of kcal) diet | 15 | 6 months | Decreased serum total PSA and proliferation rate of benign epithelium. | [63] |
| PC patients awaiting prostatectomy | Flaxseed 30 g/day combined with low-fat (≤20% of kcal) diet | 25 | average 34 days | Significant decrease in total testosterone and free androgen indices. Among men with Gleason sums of ≤ 6 decreased tumor proliferation index. Increased tumor apoptotic scores in flaxseed group compared to historic controls. | [64] |
| PC patients awaiting for prostatectomy | Control (usual) diet | 41 | average 30 days | Significantly reduced tumor proliferation rates with flaxseed supplemented diets. | [65] |
| Flaxseed 30 g/day diet | 40 | ||||
| low-fat (≤ 20% of kcal) diet | 40 | ||||
| Flaxseed 30 g/day combined with low-fat diet | 40 |
7. More Information is Needed For a Health Claim
References
- Martínez, M.E.; Giovannucci, E. Diet and the prevention of cancer. Cancer Metastasis Rev. 1997, 16, 357–376. [Google Scholar]
- McCann, M.J.; Gill, C.I.; McGlynn, H.; Rowland, I.R. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York. Nutr. Cancer. 2005, 53, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; Parsons, J.K.; Bearden, J.D., 3rd; Crawford, E.D.; Goodman, G.E.; Claudio, J.; Winquist, E.; Cook, E.D.; Karp, D.D.; Walther, P.; Lieber, M.M.; Kristal, A.R.; Darke, A.K.; Arnold, K.B.; Ganz, P.A.; Santella, R.M.; Albanes, D.; Taylor, P.R.; Probstfield, J.L.; Jagpal, T.J.; Crowley, J.J.; Meyskens, F.L. Jr; Baker, L.H.; Coltman, C.A., Jr. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [PubMed]
- Gaziano, J.M.; Glynn, R.J.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Sesso, H.D.; Buring, J.E. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA 2009, 301, 52–62. [Google Scholar]
- Peters, U.; Leitzmann, M.F.; Chatterjee, N.; Wang, Y.; Albanes, D.; Gelmann, E.P.; Friesen, M.D.; Riboli, E.; Hayes, R.B. Serum lycopene, other carotenoids, and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trail. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 962–968. [Google Scholar] [PubMed]
- Milder, I.E.; Arts, I.C.; van de Putte, B.; Venema, D.P.; Hollman, P.C. Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 2005, 93, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr. Cancer. 2006, 54, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, N.M.; Smeds, A.; Mäkelä, S.I.; Hakala, K.; Pihlava, J.M.; Ryhänen, E.L.; Sjöholm, R.; Santti, R. Structural determinants of plant lignans for the formation of enterolactone in vivo. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 777, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Peñalvo, J.L.; Heinonen, S.M.; Aura, A.M.; Adlercreutz, H. Dietary sesamin is converted to enterolactone in humans. J. Nutr. 2005, 135, 1056–1062. [Google Scholar]
- Laerke, H.N.; Mortensen, M.A.; Hedemann, M.S.; Bach Knudsen, K.E.; Penalvo, J.L.; Adlercreutz, H. Quantitative aspects of the metabolism of lignans in pigs fed fibre-enriched rye and wheat bread. Br. J. Nutr. 2009, 102, 985–994. [Google Scholar]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wähälä, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar]
- Liu, Z.; Saarinen, N.M.; Thompson, L.U. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J. Nutr. 2006, 136, 906–912. [Google Scholar]
- Travis, R.C.; Spencer, E.A.; Allen, N.E.; Appleby, P.N.; Roddam, A.W.; Overvad, K.; Johnsen, N.F.; Olsen, A.; Kaaks, R.; Linseisen, J.; Boeing, H.; Nöthlings, U.; Bueno-de-Mesquita, H.B.; Ros, M.M.; Sacerdote, C.; Palli, D.; Tumino, R.; Berrino, F.; Trichopoulou, A.; Dilis, V.; Trichopoulos, D.; Chirlaque, M.D.; Ardanaz, E.; Larranaga, N.; Gonzalez, C.; Suárez, L.R.; Sánchez, M.J.; Bingham, S.; Khaw, K.T.; Hallmans, G.; Stattin, P.; Rinaldi, S.; Slimani, N.; Jenab, M.; Riboli, E.; Key, T.J. Plasma phyto-oestrogens and prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer. 2009, 100, 1817–1823. [Google Scholar] [PubMed]
- Strom, S.S.; Yamamura, Y.; Duphorne, C.M.; Spitz, M.R.; Babaian, R.J.; Pillow, P.C.; Hursting, S.D. Phytoestrogen intake and prostate cancer: a case-control study using a new database. Nutr. Cancer. 1999, 33, 20–25. [Google Scholar]
- Hedelin, M.; Klint, A.; Chang, E.T.; Bellocco, R.; Johansson, J.E.; Andersson, S.O.; Heinonen, S.M.; Adlercreutz, H.; Adami, H.O.; Grönberg, H.; Bälter, K.A. Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: the cancer prostate Sweden study (Sweden). Cancer Causes Control. 2006, 17, 169–180. [Google Scholar]
- Stattin, P.; Adlercreutz, H.; Tenkanen, L.; Jellum, E.; Lumme, S.; Hallmans, G.; Harvei, S.; Teppo, L.; Stumpf, K.; Luostarinen, T.; Lehtinen, M.; Dillner, J.; Hakama, M. Circulating enterolactone and prostate cancer risk: a Nordic nested case-control study. Int. J. Cancer. 2002, 99, 124–129. [Google Scholar]
- Kilkkinen, A.; Virtamo, J.; Virtanen, M.J.; Adlercreutz, H.; Albanes, D.; Pietinen, P. Serum enterolactone concentration is not associated with prostate cancer risk in a nested case-control study. Cancer Epidemiol. Biomarkers Prev. 2003, 12, 1209–1212. [Google Scholar]
- Stattin, P.; Bylund, A.; Biessy, C.; Kaaks, R.; Hallmans, G.; Adlercreutz, H. Prospective study of plasma enterolactone and prostate cancer risk (Sweden). Cancer Causes Control. 2004, 15, 1095–1102. [Google Scholar]
- Heald, C.L.; Ritchie, M.R.; Bolton-Smith, C.; Morton, M.S.; Alexander, F.E. Phyto-oestrogens and risk of prostate cancer in Scottish men. Br. J. Nutr. 2007, 98, 388–396. [Google Scholar]
- Ward, H.; Chapelais, G.; Kuhnle, G.G.; Luben, R.; Khaw, K.T.; Bingham, S. Lack of prospective associations between plasma and urinary phytoestrogens and risk of prostate or colorectal cancer in the European Prospective into Cancer-Norfolk study. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 2891–2894. [Google Scholar]
- Hausner, H.; Johnsen, N.F.; Hallund, J.; Tetens, I. A single measurement is inadequate to estimate enterolactone levels in Danish postmenopausal women due to large intraindividual variation. J. Nutr. 2004, 134, 1197–1200. [Google Scholar]
- Bylund, A.; Saarinen, N.; Zhang, J.X.; Bergh, A.; Widmark, A.; Johansson, A.; Lundin, E.; Adlercreutz, H.; Hallmans, G.; Stattin, P.; Mäkelä, S. Anticancer effects of a plant lignan 7-hydroxymatairesinol on a prostate cancer model in vivo. Exp. Biol. Med. (Maywood). 2005, 230, 217–223. [Google Scholar] [PubMed]
- Mazur, W.M.; Uehara, M.; Wähälä, K.; Adlercreutz, H. Phyto-oestrogen content of berries, and plasma concentrations and urinary excretion of enterolactone after a single strawberry-meal in human subjects. Br. J. Nutr. 2000, 83, 381–387. [Google Scholar]
- Saarinen, N.M.; Tuominen, J.; Santti, R.; Pylkkänen, L. Bromacology: Pharmacology of Foods and Their Components; Yagasaki, K., Yamazaki, M., Eds.; Research Signpost, Trivandrum: Kerala, India, 2008; p. 30. Chapter 1. [Google Scholar]
- Hallund, J.; Tetens, I.; Bügel, S.; Tholstrup, T.; Ferrari, M.; Teerlink, T.; Kjaer, A.; Wiinberg, N. Daily consumption for six weeks of a lignan complex isolated from flaxseed does not affect endothelial function in healthy postmenopausal women. J. Nutr. 2006, 136, 2314–2318. [Google Scholar]
- Kuijsten, A.; Arts, I.C.; Vree, T.B.; Hollman, PC. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J. Nutr. 2005, 135, 795–801. [Google Scholar]
- Lin, X.; Gingrich, J.R.; Bao, W.; Li, J.; Haroon, Z.A.; Demark-Wahnefried, W. Effect of flaxseed supplementation on prostatic carcinoma in transgenic mice. Urology 2002, 60, 919–924. [Google Scholar]
- Greenberg, N.M.; DeMayo, F.J.; Sheppard, P.C.; Barrios, R.; Lebovitz, R.; Finegold, M.; Angelopoulou, R.; Dodd, J.G.; Duckworth, M.L.; Rosen, J.M. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol. Endocrinol. 1994, 8, 230–239. [Google Scholar] [Green Version]
- Greenberg, N.M.; DeMayo, F; Finegold, M.J.; Medina, D.; Tilley, W.D.; Aspinall, J.O.; Cunha, G.R.; Donjacour, A.A.; Matusik, R.J.; Rosen, J.M. Prostate cancer in a transgenic mouse. Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 3439–3443. [Google Scholar] [PubMed][Green Version]
- Kaplan-Lefko, P.J.; Chen, T.M.; Ittmann, M.M.; Barrios, R.J.; Ayala, G.E.; Huss, W.J.; Maddison, L.A.; Foster, B.A.; Greenberg, N.M. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate 2003, 55, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Thompson, L.U.; Rickard, S.E.; Cheung, F.; Kenaschuk, E.O.; Obermeyer, W.R. Variability in anticancer lignan levels in flaxseed. Nutr. Cancer 1997, 27, 26–30. [Google Scholar]
- Hyvärinen, H.K.; Pihlava, J.M.; Hiidenhovi, J.A.; Hietaniemi, V.; Korhonen, H.J.; Ryhänen, E.L. Effect of processing and storage on the stability of flaxseed lignan added to dairy products. J. Agric. Food Chem. 2006, 54, 8788–8792. [Google Scholar]
- Lin, X.; Switzer, B.R.; Demark-Wahnefried, W. Effect of mammalian lignans on the growth of prostate cancer cell lines. Anticancer Res. 2001, 21, 3995–3999. [Google Scholar]
- McCann, M.J.; Gill, C.I.; Linton, T.; Berrar, D.; McGlynn, H.; Rowland, I.R. Enterolactone restricts the proliferation of the LNCaP human prostate cancer cell line in vitro. Mol. Nutr Food Res. 2008, 52, 567–580. [Google Scholar]
- Chen, L.H.; Fang, J.; Sun, Z.; Li, H.; Wu, Y.; Demark-Wahnefried, W.; Lin, X. Enterolactone inhibits insulin-like growth factor-1 receptor signaling in human prostatic carcinoma PC-3 cells. J. Nutr. 2009, 139, 653–659. [Google Scholar]
- Saarinen, N.M.; Wärri, A.; Dings, R.P.; Airio, M.; Smeds, A.I.; Mäkelä, S. Dietary lariciresinol attenuates mammary tumor growth and reduces blood vessel density in human MCF-7 breast cancer xenografts and carcinogen-induced mammary tumors in rats. Int. J. Cancer. 2008, 23, 1196–1204. [Google Scholar]
- Miura, D.; Saarinen, N.M.; Miura, Y.; Santti, R.; Yagasaki, K. Hydroxymatairesinol and its mammalian metabolite enterolactone reduce the growth and metastasis of subcutaneous AH109A hepatomas in rats. Nutr. Cancer 2007, 58, 49–59. [Google Scholar] [PubMed]
- Danbara, N.; Yuri, T.; Tsujita-Kyutoku, M.; Tsukamoto, R.; Uehara, N.; Tsubura, A. Enterolactone induces apoptosis and inhibits growth of Colo 201 human colon cancer cells both in vitro and in vivo. Anticancer Res. 2005, 25, 2269–2276. [Google Scholar]
- Chen, L.H.; Fang, J.; Li, H.; Demark-Wahnefried, W.; Lin, X. Enterolactone induces apoptosis in human prostate carcinoma LNCaP cells via a mitochondrial-mediated, caspase-dependent pathway. Mol. Cancer Ther. 2007, 6, 2581–2590. [Google Scholar]
- Peuhu, E.; Rivero-Müller, A.; Stykki, H.; Torvaldson, E.; Holmbom, T.; Eklund, P.; Sjöholm, R.; Eriksson, J.E.; Unkila, M. Inhibition of Akt signaling by the lignan matairesinol sensitizes prostate cancer cells to TRAIL-induced apoptosis. Oncogene 2009, in press. [Google Scholar]
- Han, H.Y.; Wang, X.H.; Wang, N.L.; Ling, M.T.; Wong, Y.C.; Yao, X.S. Lignans isolated from Campylotropis hirtella (Franch.) Schindl. Decreased prostate specific antigen and androgen receptor expression in LNCaP cells. J. Agric. Food Chem. 2008, 56, 6928–6935. [Google Scholar] [PubMed]
- Adlercreutz, H. Lignans and human health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar]
- Cosentino, M.; Marino, F.; Ferrari, M.; Rasini, E.; Bombelli, R.; Luini, A.; Legnaro, M.; Delle Canne, M.G.; Luzzani, M.; Crema, F.; Paracchini, S.; Lecchini, S. Estrogenic activity of 7-hydroxymatairesinol potassium acetate (HMR/lignan) from Norway spruce (Picea abies) knots and of its active metabolite enterolactone in MCF-7 cells. Pharmacol. Res. 2007, 56, 140–147. [Google Scholar]
- Ellem, S.J.; Wang, H.; Poutanen, M.; Risbridger, G.P. Increased endogenous estrogen synthesis leads to the sequential induction of prostatic inflammation (prostatitis) and prostatic pre-malignancy. Amer. J. Pathol. 2009, 175, 1187–1199. [Google Scholar]
- Prins, G.S.; Korach, K.S. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids 2008, 73, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Penttinen, P.; Jaehrling, J.; Damdimopoulos, A.E.; Inzunza, J.; Lemmen, J.G.; van der Saag, P.; Pettersson, K.; Gauglitz, G.; Mäkelä, S.; Pongratz, I. Diet-derived polyphenol metabolite enterolactone is a tissue-specific estrogen receptor activator. Endocrinology. 2007, 148, 4875–4886. [Google Scholar]
- Saarinen, N.M.; Wärri, A.; Mäkelä, S.I.; Eckerman, C.; Reunanen, M.; Ahotupa, M.; Salmi, S.M.; Franke, A.A.; Kangas, L.; Santti, R. Hydroxymatairesinol, a novel enterolactone precursor with antitumor properties from coniferous tree (Picea abies). Nutr. Cancer 2000, 36, 207–216. [Google Scholar]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar]
- Setchell, K.D.; Lawson, A.M.; Borriello, S.P.; Harkness, R.; Gordon, H.; Morgan, D.M.; Kirk, D.N.; Adlercreutz, H.; Anderson, L.C.; Axelson, M. Lignan formation in man--microbial involvement and possible roles in relation to cancer. Lancet 1981, 2, 4–7. [Google Scholar]
- Tou, J.C.; Chen, J.; Thompson, L.U. Flaxseed and its lignan precursor, secoisolariciresinol diglycoside, affect pregnancy outcome and reproductive development in rats. J. Nutr. 1998, 128, 1861–1868. [Google Scholar] [PubMed]
- Evans, B.A.; Griffiths, K.; Morton, M.S. Inhibition of 5 alpha-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J. Endocrinol. 1995, 147, 295–302. [Google Scholar]
- Takeuchi, S.; Takahashi, T.; Sawada, Y.; Iida, M.; Matsuda, T.; Kojima, H. Comparative study on the nuclear hormone receptor activity of various phytochemicals and their metabolites by reporter gene assays using Chinese hamster ovary cells. Biol. Pharm. Bull. 2009, 32, 195–202. [Google Scholar]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell Biochem. 1999, 202, 91–100. [Google Scholar]
- Kangas, L.; Saarinen, N.; Mutanen, M.; Ahotupa, M.; Hirsinummi, R.; Unkila, M.; Perälä, M.; Soininen, P.; Laatikainen, R.; Korte, H.; Santti, R. Antioxidant and antitumor effects of hydroxymatairesinol (HM-3000, HMR), a lignan isolated from the knots of spruce. Eur. J. Cancer Prev. 2002, 2, S48–S57. [Google Scholar]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar]
- Yamashita, K.; Ikeda, S.; Obayashi, M. Comparative effects of flaxseed and sesame seed on vitamin E and cholesterol levels in rats. Lipids 2003, 38, 1249–1255. [Google Scholar]
- Loeb, S.; Partin, A.W. Randomized trials of selenium, vitamin e, or vitamin C for prostate cancer prevention. Rev. Urol. 2009, 11, 114–115. [Google Scholar] [PubMed]
- Kelloff, G.J.; Higley, H.R.; Brawer, M.K.; Lucia, M.S.; Sigman, C.C.; Crawford, E.D. Chemoprevention strategies in the prostate: an overview. Rev. Urol. 2002, 4, 69–77. [Google Scholar]
- Fleshner, N.E.; Lawrentschuk, N. Risk of developing prostate cancer in the future: overview of prognostic biomarkers. Urology 2009, 73, S21–S27. [Google Scholar]
- Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Miller, G.J.; Ford, L.G.; Lieber, M.M.; Cespedes, R.D.; Atkins, J.N.; Lippman, S.M.; Carlin, S.M.; Ryan, A.; Szczepanek, C.M.; Crowley, J.J.; Coltman, C.A., Jr. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 2003, 349, 215–224. [Google Scholar]
- Nelson, W.G. Agents in development for prostate cancer prevention. Expert Opin. Investig. Drugs. 2004, 13, 1541–1554. [Google Scholar]
- Demark-Wahnefried, W.; Robertson, C.N.; Walther, P.J.; Polascik, T.J.; Paulson, D.F.; Vollmer, R.T. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate-specific antigen. Urology 2004, 63, 900–904. [Google Scholar]
- Demark-Wahnefried, W.; Price, D.T.; Polascik, T.J.; Robertson, C.N.; Anderson, E.E.; Paulson, D.F.; Walther, P.J.; Gannon, M.; Vollmer, R.T. Pilot study of dietary fat restriction and flaxseed supplementation in men with prostate cancer before surgery: exploring the effects on hormonal levels, prostate-specific antigen, and histopathologic features. Urology 2001, 58, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Polascik, T.J.; George, S.L.; Switzer, B.R.; Madden, J.F.; Ruffin, M.T., 4th; Snyder, D.C.; Owzar, K.; Hars, V.; Albala, D.M.; Walther, P.J.; Robertson, C.N.; Moul, J.W.; Dunn, B.K.; Brenner, D.; Minasian, L.; Stella, P.; Vollmer, R.T. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 3577–3587. [Google Scholar] [PubMed]
- Samarasekera, V. Universities need a new social contract. Nature 2009, 462, 160–161. [Google Scholar]
- Wiygul, J.B.; Evans, B.R.; Peterson, B.L.; Polascik, T.J.; Walther, P.J.; Robertson, C.N.; Albala, D.M.; Demark-Wahnefried, W. Supplement use among men with prostate cancer. Urology 2005, 66, 161–166. [Google Scholar]
- Park, S.Y.; Wilkens, L.R.; Franke, A.A.; Le Marchand, L.; Kakazu, K.K.; Goodman, M.T.; Murphy, S.P; Henderson, B.E.; Kolonel, L.N. Urinary phytoestrogen excretion and prostate cancer risk: a nested case-control study in the Multiethnic Cohort. Br. J. Cancer. 2009, 101, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, R.; Thomas, K.; Grace, P.; Dearnaley, D.; Horwich, A.; Huddart, R.; Parker, C.C. Baseline urinary phytoestrogen levels and the natural history of untreated, localised prostate cancer in a British population. Int. J. Biol. Markers. 2008, 23, 192–197. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saarinen, N.M.; Tuominen, J.; Pylkkänen, L.; Santti, R. Assessment of Information to Substantiate a Health Claim on the Prevention of Prostate Cancer by Lignans. Nutrients 2010, 2, 99-115. https://doi.org/10.3390/nu2020099
Saarinen NM, Tuominen J, Pylkkänen L, Santti R. Assessment of Information to Substantiate a Health Claim on the Prevention of Prostate Cancer by Lignans. Nutrients. 2010; 2(2):99-115. https://doi.org/10.3390/nu2020099
Chicago/Turabian StyleSaarinen, Niina M., Juhani Tuominen, Liisa Pylkkänen, and Risto Santti. 2010. "Assessment of Information to Substantiate a Health Claim on the Prevention of Prostate Cancer by Lignans" Nutrients 2, no. 2: 99-115. https://doi.org/10.3390/nu2020099
APA StyleSaarinen, N. M., Tuominen, J., Pylkkänen, L., & Santti, R. (2010). Assessment of Information to Substantiate a Health Claim on the Prevention of Prostate Cancer by Lignans. Nutrients, 2(2), 99-115. https://doi.org/10.3390/nu2020099




