SU-8 Photolithography as a Toolbox for Carbon MEMS
Abstract
:1. Introduction
2. Photolithography of SU-8
2.1. Releasable Substrate for Free-Standing Parts
2.2. Dense Arrays of High Aspect Ratio Structures
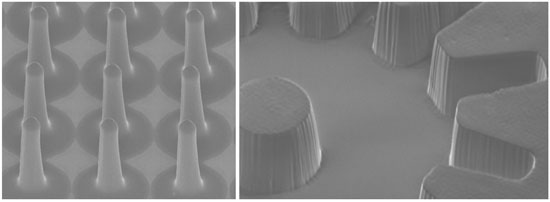
2.3. Backexposure for Tapered Structures
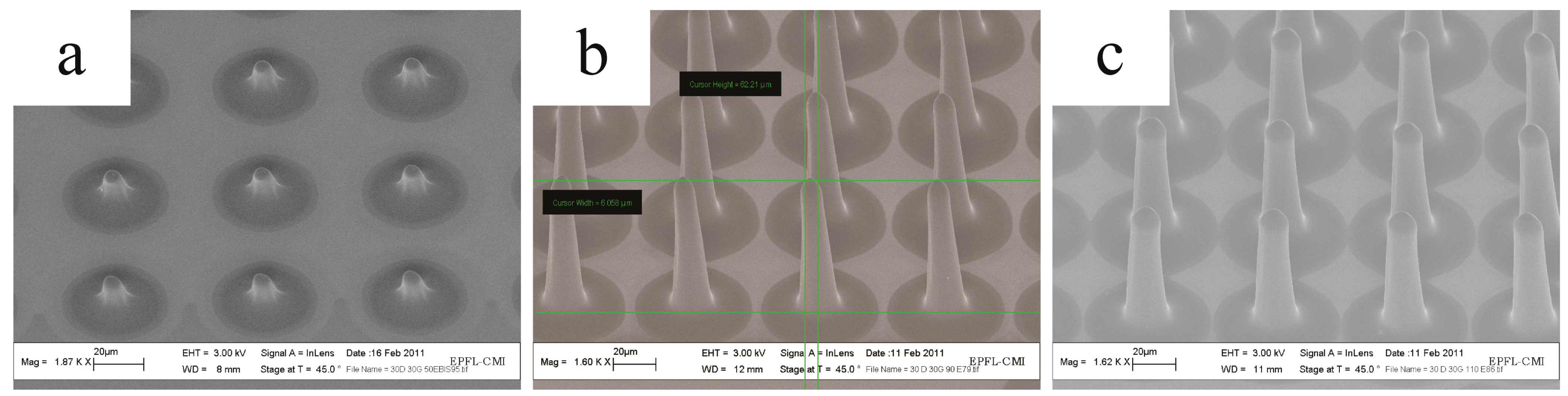
2.4. Grayscale Photolithography

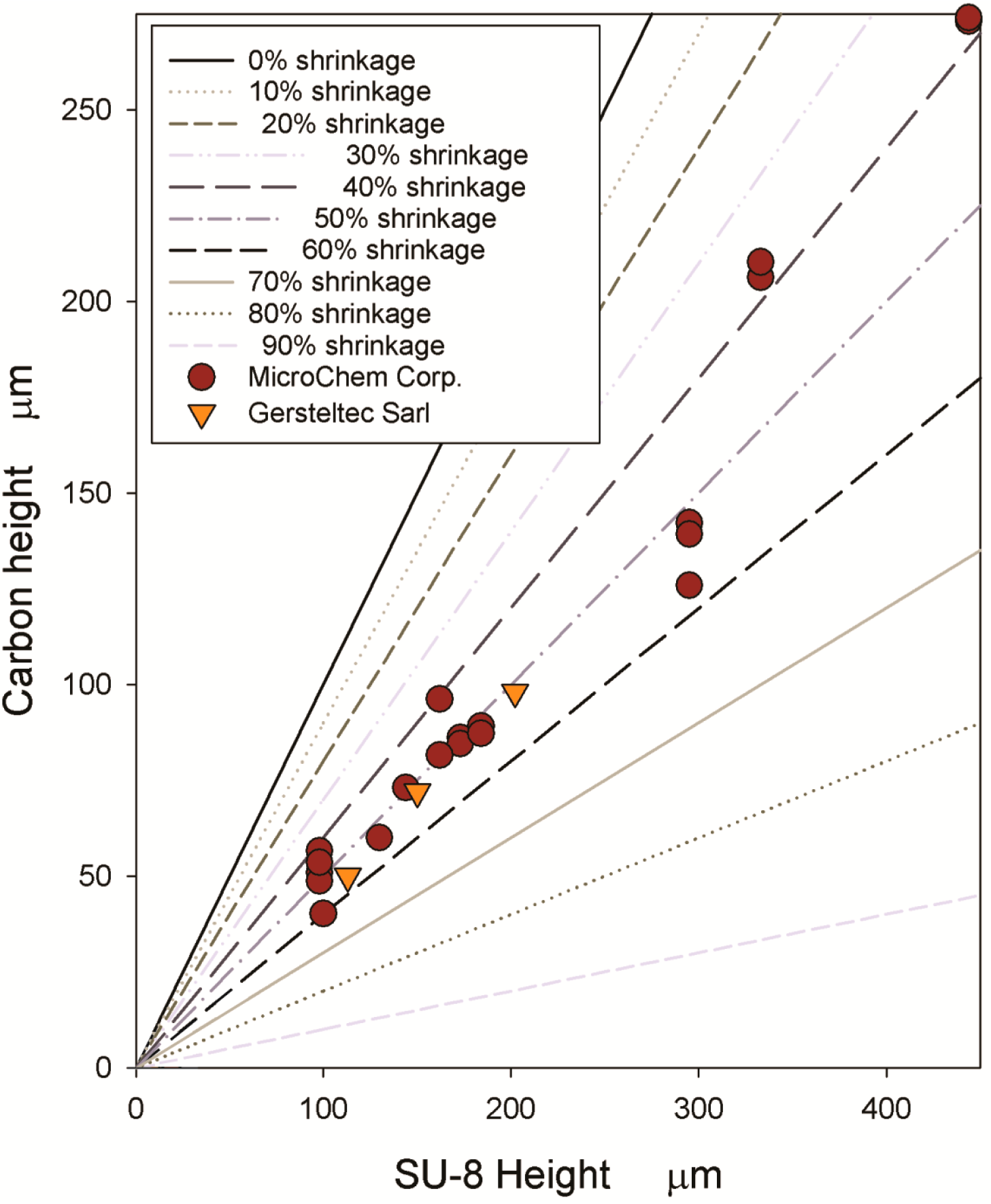
3. Carbonization
4. Selected Applications
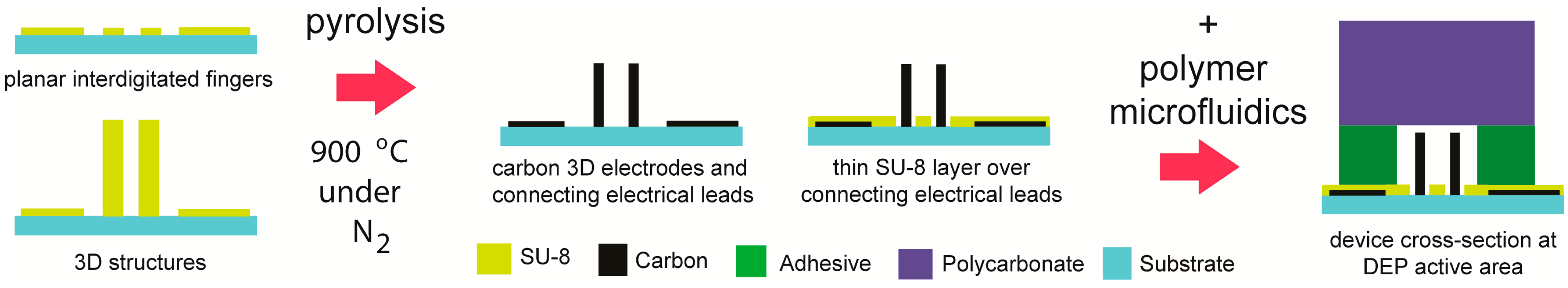
4.1. Carbon-Electrode Dielectrophoresis
4.2. Carbon Micromolds
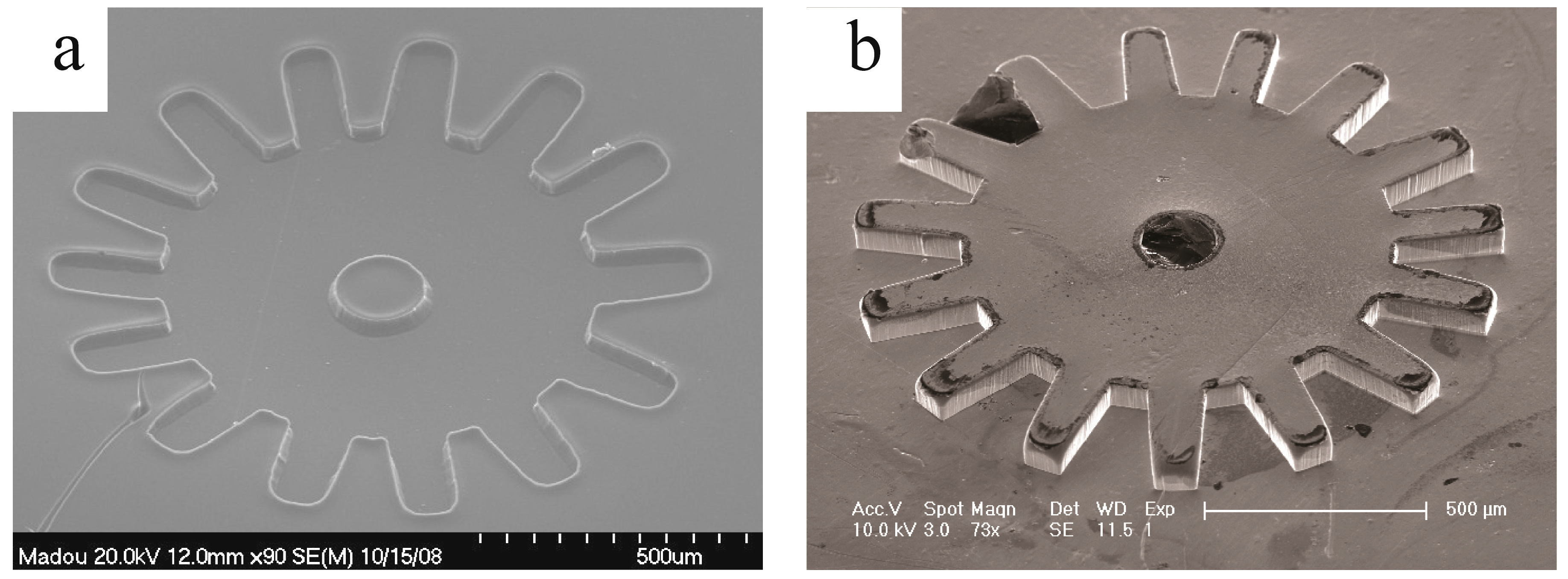
5. Conclusions
Supplementary Materials
Acknowledgements
Conflicts of Interest
References
- Jenkins, G.; Kawamura, K. Structure of glassy carbon. Nature 1971, 231, 175–176. [Google Scholar] [CrossRef]
- Pesin, L.A. Structure and properties of glass-like carbon. J. Mater. Sci. 2002, 37, 1–28. [Google Scholar] [CrossRef]
- Jenkins, G.; Kawamura, K.; Ban, L. Formation and structure of polymeric carbons. Proc. R. Soc. Lond. A Math. Phys. Sci. 1972, 327, 501–517. [Google Scholar] [CrossRef]
- Kakinoki, J. A model for the structure of “glassy carbon”. Acta Crystallogr. 1965, 18, 578. [Google Scholar] [CrossRef]
- Fitzer, E.; Kochling, K.H.; Boehm, H.P.; Marsh, H. Recommended terminology for the desciption of carbon as a solid. Pure Appl. Chem. 1995, 67, 473–506. [Google Scholar] [CrossRef]
- Zittel, H.E.; Miller, F.J.A. Glassy-carbon electrode for voltammetry. Anal. Chem. 1965, 37, 200–203. [Google Scholar] [CrossRef]
- Van der Linde, W.E.; Dieker, J.W. Glassy carbon as electrode material in electro-analytical chemistry. Anal. Chim. Acta 1980, 119, 1–24. [Google Scholar]
- Yamada, S.; Sato, H. Some physical properties of glassy carbon. Nature 1962, 193, 261–262. [Google Scholar] [CrossRef]
- Rothwell, W. Small-angle X-ray scattering from glassy carbon. J. Appl. Phys. 1968, 39, 1840–1845. [Google Scholar] [CrossRef]
- McFeely, S.P.; Kowalczyk, S.P.; Ley, L.; Cavell, R.G.; Pollak, R.A.; Shirley, D.A. X-ray photoemission studies of diamond, graphite, and glassy carbon valence bands. Phys. Rev. B 1974, 9, 5268–5278. [Google Scholar] [CrossRef]
- Pesin, L.A.; Baitinger, E.M. A new structural model of glass-like carbon. Carbon 2002, 40, 295–306. [Google Scholar] [CrossRef]
- Yoshida, A.; Kaburagi, Y.; Hishiyama, Y. Microtexture and magnetoresistance of glass-like carbons. Carbon 1991, 29, 1107–1111. [Google Scholar] [CrossRef]
- Spain, I. Electronic Transport properties of graphite, carbons, and related materials. In Chemistry and Physics of Carbon; Walker, P.L., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1981; pp. 119–304. [Google Scholar]
- Ranganathan, S.; McCreery, R.; Majji, S.M.; Madou, M. Photoresist-derived carbon for microelectromechanical systems and electrochemical applications. J. Electrochem. Soc. 2000, 147, 277–282. [Google Scholar] [CrossRef]
- Gelorme, J.; Cox, R.; Gutierrez, S. Photoresist Composition and Printed Circuit Boards and Packages Made Therewith. U.S. Patent 4,882,245, 21 November 1989. [Google Scholar]
- Singh, A.; Jayaram, J.; Madou, M.; Akbar, S. Pyrolysis of negative photoresists to fabricate carbon structures for microelectromechanical systems and electrochemical applications. J. Electrochem. Soc. 2002, 149, E78–E83. [Google Scholar] [CrossRef]
- Wang, C.; Jia, G.; Taherabadi, L.H.; Madou, M.J. A novel method for the fabrication of high-aspect ratio C-MEMS structures. J. Microelectromech. Syst. 2005, 14, 348–358. [Google Scholar] [CrossRef]
- Wang, C.; Madou, M. From MEMS to NEMS with carbon. Biosens. Bioelectron. 2005, 20, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Amato, L.; Keller, S.S.; Heiskanen, A.; Dimaki, M.; Emnéus, J.; Boisen, A.; Tenje, M. Fabrication of high-aspect ratio SU-8 micropillar arrays. Microelectron. Eng. 2012, 98, 483–487. [Google Scholar] [CrossRef]
- Heo, J.I.; Shim, D.S.; Teixidor, G.T.; Oh, S.; Madou, M.J.; Shin, H. Carbon interdigitated array nanoelectrodes for electrochemical applications. J. Electrochem. Soc. 2011, 158, J76–J80. [Google Scholar] [CrossRef]
- Martinez-Duarte, R. Fabrication of Carbon Micro Molds; University of California: Irvine, CA, USA, 2009. [Google Scholar]
- Martinez-Duarte, R.; Madou, M.J.; Kumar, G.; Schroers, J. A novel method for amorphous metal micromolding using carbon MEMS. In Proceedings of Solid-State Sensors, Actuators and Microsystems Conference, Denver, CO, USA, 21–25 June 2009; pp. 188–191.
- Malladi, K.; Wang, C.; Madou, M. Fabrication of suspended carbon microstructures by E-beam writer and pyrolysis. Carbon 2006, 44, 2602–2607. [Google Scholar] [CrossRef]
- Martinez-Duarte, R.; Gorkin, R.A.; Abi-Samra, K.; Madou, M.J. The integration of 3D carbon-electrode dielectrophoresis on a CD-like centrifugal microfluidic platform. Lab Chip 2010, 10, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Abgrall, P.; Conedera, V.; Camon, H.; Gue, A.M.; Nguyen, N.T. SU-8 as a structural material for labs-on-chips and microelectromechanical systems. Electrophoresis 2007, 28, 4539–4551. [Google Scholar] [CrossRef] [PubMed]
- Campo, A.; del Greiner, C. SU-8: A photoresist for high-aspect-ratio and 3D submicron lithography. J. Micromech. Microeng. 2007, 17, R81–R95. [Google Scholar] [CrossRef]
- Martinez-Duarte, R.; Madou, M. SU-8 photolithography and its impact on microfluidics. In Microfluidics and Nanofluidics Handbook: Fabrication, Implementation and Applications; Mitra, S., Chakraborty, S., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 231–268. [Google Scholar]
- Abgrall, P.; Lattes, C.; Conédéra, V.; Dollat, X.; Colin, S.; Gué, A.M. A novel fabrication method of flexible and monolithic 3D microfluidic structures using lamination of SU-8 films. J. Micromech. Microeng. 2005, 16, 113–121. [Google Scholar] [CrossRef]
- Yang, R.; Wang, W. A numerical and experimental study on gap compensation and wavelength selection in UV-lithography of ultra-high aspect ratio SU-8 microstructures. Sens. Actuators B Chem. 2005, 110, 279–288. [Google Scholar] [CrossRef]
- Chuang, Y.J.; Tseng, F.G.; Lin, W.K. Reduction of diffraction effect of UV exposure on SU-8 negative thick photoresist by air gap elimination. Microsyst. Technol. 2002, 8, 308–313. [Google Scholar] [CrossRef]
- Martinez-Duarte, R.; Renaud, P.; Madou, M. A novel approach to dielectrophoresis using carbon electrodes. Electrophoresis 2011, 32, 2385–2392. [Google Scholar] [PubMed]
- Martinez-Duarte, R. Label-Free Cell Sorting Using Carbon-Electrode Dielectrophoresis and Centrifugal Microfluidics; University of California: Irvine, CA, USA, 2010. [Google Scholar]
- Zhang, J.; Chan-Park, M.B.; Conner, S.R. Effect of exposure dose on the replication fidelity and profile of very high aspect ratio microchannels in SU-8. Lab Chip 2004, 4, 646–653. [Google Scholar] [CrossRef]
- Windt, D. L.; Cirelli, R.A. Amorphous carbon films for use as both variable-transmission apertures and attenuated phase shift masks for deep ultraviolet lithography. J. Vac. Sci. Technol. B. 1999, 17, 930–932. [Google Scholar] [CrossRef]
- Sure, A.; Dillon, T.; Murakowski, J.; Lin, C.; Pustai, D.; Prather, D. Fabrication and characterization of three-dimensional silicon tapers. Opt. Express 2003, 11, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Taff, J.; Kashte, Y. Fabricating multilevel SU-8 structures in a single photolithographic step using colored masking patterns. J. Vac. Sci. Technol. A 2006, 24, 742–746. [Google Scholar] [CrossRef]
- Hirai, Y.; Inamoto, Y.; Sugano, K.; Tsuchiya, T.; Tabata, O. Moving mask UV lithography for three-dimensional structuring. J. Micromech. Microeng. 2007, 17, 199–206. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Park, J.H.; Allen, M.G. Multidirectional UV lithography for complex 3-D MEMS structures. J. Microelectromech. Syst. 2006, 15, 1121–1130. [Google Scholar] [CrossRef]
- Ullal, C.K.; Maldovan, M.; Thomas, E.L.; Chen, G.; Han, Y.J.; Yang, S. Photonic crystals through holographic lithography: Simple cubic, diamond-like, and gyroid-like structures. Appl. Phys. Lett. 2004, 84. [Google Scholar] [CrossRef]
- Deubel, M.; von Freymann, G.; Wegener, M.; Pereira, S.; Busch, K.; Soukoulis, C.M. Direct laser writing of three-dimensional photonic-crystal templates for telecommunications. Nat. Mater. 2004, 3, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, A.; Lorenz, H.; Renaud, P. 3D microfabrication by combining microstereolithography and thick resist UV lithography. Sens. Actuators A Phys. 1999, 73, 14–23. [Google Scholar] [CrossRef]
- Rammohan, A.; Dwivedi, P.K.; Martinez-Duarte, R.; Katepalli, H.; Madou, M.J.; Sharma, A. One-step maskless grayscale lithography for the fabrication of 3-dimensional structures in SU-8. Sens. Actuators B Chem. 2011, 153, 125–134. [Google Scholar] [CrossRef]
- Nishikawa, K.; Fukuyama, K.; Nishizawa, T. Structure change of glass-like carbon with heat treatment, studied by small angle X-ray scattering: I. Glass-like carbon prepared from phenolic resin. Jpn. J. Appl. Phys. 1998, 37, 6486–6491. [Google Scholar]
- Jenkins, G.M.; Kawamura, K. Polymeric Carbon—Carbon Fibre, Glass and Char; Cambridge University Press: London, UK, 1976. [Google Scholar]
- Fitzer, E.; Schaefer, W.; Yamada, S. The Formation of glasslike carbon by pyrolysis of polyfurfuryl alcohol and phenolic resin. Carbon 1969, 7, 643–648. [Google Scholar] [CrossRef]
- Vohler, O.; Reiser, P.; Martina, R.; Overhoff, D. New forms of carbon. Angew Chem. Int. Ed. 1970, 9, 414–425. [Google Scholar] [CrossRef]
- Kawamura, K.; Kimura, S. Glass-like carbon made from epoxy resin cured with 2,4,6-trinitrophenol. Bull. Chem. Soc. Jpn. 1983, 56, 2499–2503. [Google Scholar] [CrossRef]
- Bhatia, G.; Aggarwal, R.; Malik, M.; Bahl, O. Conversion of phenol formaldehyde resin to glass-like carbon. J. Mater. Sci. 1984, 19, 1022–1028. [Google Scholar] [CrossRef]
- Neenan, T.; Callstrom, M.; Bachman, B.; McCreery, R.L.; Alsmeyer, D.C. Doped glassy carbon materials (DGC): Their synthesis from polymeric precursors and investigation of their properties. Br. Polym. J. 1990, 23, 171–177. [Google Scholar] [CrossRef]
- Schueller, O.J.A.; Brittain, S.T.; Whitesides, G.M. Fabrication of glassy carbon microstructures by pyrolysis of microfabricated polymeric precursors. Adv. Mater. 1997, 9, 477–480. [Google Scholar] [CrossRef]
- Shah, H.V.; Brittain, S.T.; Huang, Q.; Hwu, S.J.; Whitesides, G.; Smith, D.W. Bis-o-diynylarene (BODA) derived polynaphtalenes as precursors to glassy carbon microstructures. Chem. Mater. 1999, 11, 9078–9079. [Google Scholar]
- Hishiyama, Y.; Igarashi, K.; Kanaoka, I.; Fujii, H. Graphitization behavior of kapton-derived carbon film related to structure, microtexture and transport properties. Carbon 1997, 35, 657–668. [Google Scholar] [CrossRef]
- Gac, N.A.; Spokes, G.N.; Benson, S.W. Thermal degradation of nadic methyl anhydride-cured epoxy novolac. J. Polym. Sci. A 1970, 8, 593–608. [Google Scholar] [CrossRef]
- Fitzer, E.; Schäfer, W. The effect of crosslinking on the formation of glasslike carbons from thermosetting resins. Carbon 1970, 8, 353–364. [Google Scholar] [CrossRef]
- Lee, L.H. Mechanisms of thermal degradation of phenolic condensation polymers. II. Thermal stability and degradation schemes of epoxy resins. J. Polym. Sci. Part A 1965, 3, 859–882. [Google Scholar]
- Lyons, A.; Wilkins, C.; Robbins, M. Thin pinhole-free carbon films. Thin Solid Films 1983, 103, 333–341. [Google Scholar] [CrossRef]
- Nakagawa, H.; Tsuge, S. Studies on thermal degradation of epoxy resins by high-resolution pyrolysis-gas chromatography. J. Anal. Appl. Pyrolysis 1987, 12, 97–113. [Google Scholar] [CrossRef]
- Chen, K.S.; Yeh, R.Z. Pyrolysis kinetics of epoxy resin in a nitrogen stmosphere. J. Hazard. Mater. 1996, 49, 105–113. [Google Scholar] [CrossRef]
- Beyler, C.; Hirschler, M. Thermal decomposition of polymers. In SFPE Handbook of Fire Protection Engineering; National Fire Protection Association: Quincy, MA, USA, 2002; pp. 110–131. [Google Scholar]
- Ma, C.C.M.; Chen, C.Y.; Kuan, H.C.; Chang, W.C. Processability, thermal, mechanical, and morphological properties of novolac type-epoxy resin-based carbon-carbon composite. J. Compos. Mater. 2004, 38, 311–322. [Google Scholar] [CrossRef]
- Mehrotra, B.; Bragg, R.; Rao, A. Effect of heat treatment temperature (HTT) on density, weight and volume of glass-like carbon (GC). J. Mater. Sci. 1983, 18, 2671–2678. [Google Scholar] [CrossRef]
- Park, B.; Taherabadi, L.; Wang, C.; Zoval, J.; Madou, M.J. Electrical properties and shrinkage of carbonized photoresist films and the implications for carbon microelectromechanical systems devices in conductive media. J. Electrochem. Soc. 2005, 152, J136–J143. [Google Scholar] [CrossRef]
- Beidaghi, M.; Chen, W.; Wang, C. Electrochemically activated carbon micro-electrode arrays for electrochemical micro-capacitors. J. Power Sources 2011, 196, 2403–2409. [Google Scholar] [CrossRef]
- Min, H.S.; Park, B.Y.; Taherabadi, L.; Wang, C.; Yeh, Y.; Zaouk, R.; Madou, M.J.; Dunn, B. Fabrication and properties of a carbon/polypyrrole three-dimensional microbattery. J. Power Sources 2008, 178, 795–800. [Google Scholar] [CrossRef]
- Teixidor, G.T.; Zaouk, R.B.; Park, B.Y.; Madou, M.J. Fabrication and characterization of three-dimensional carbon electrodes for lithium-ion batteries. J. Power Sources 2008, 183, 730–740. [Google Scholar] [CrossRef]
- Martinez-Duarte, R. Microfabrication technologies in dielectrophoresis applications—A review. Electrophoresis 2012, 33, 3110–3132. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.D.C.; Torrents, E.; Martinez-Duarte, R.; Madou, M.J.; Juarez, A. On-line separation of bacterial cells by carbon-electrode dielectrophoresis. Electrophoresis 2010, 31, 2921–2928. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.D.C.; Martínez-Duarte, R.; Hüttener, M.; Renaud, P.; Torrents, E.; Juárez, A. Increasing PCR densitivity by removal of polymerase inhibitors in environmental samples by using dielectrophoresis. Biosens. Bioelectron. 2013, 43, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Elitas, M.; Martinez-Duarte, R.; Dhar, N.; McKinney, J.D.; Renaud, P. Dielectrophoresis-based purification of antibiotic-treated bacterial subpopulations. Lab Chip 2014, 14, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Duarte, R.; Camacho-Alanis, F.; Renaud, P.; Ros, A. Dielectrophoresis of lambda-DNA using 3D carbon electrodes. Electrophoresis 2013, 34, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Mernier, G.; Martinez-Duarte, R.; Lehal, R.; Radtke, F.; Renaud, P. Very high throughput electrical cell lysis and extraction of intracellular compounds using 3D carbon electrodes in lab-on-a-chip devices. Micromachines 2012, 3, 574–581. [Google Scholar] [CrossRef]
- Schroers, J.; Kumar, G.; Madou, M.; Martinez-Duarte, R. Carbon Molds for Use in the Fabrication of Bulk Metallic Glass Parts and Molds. U.S. Patent 2,012,0125,071 A1, 24 May 2012. [Google Scholar]
- Telford, M. The case for bulk metallic glass. Mater. Today 2004, 7, 36–43. [Google Scholar] [CrossRef]
- Johnson, W.L. Bulk amorphous metal—An emerging engineering material. JOM 2002, 54, 40–43. [Google Scholar] [CrossRef]
- Schroers, J.; Pham, Q.; Desai, A. Thermoplastic forming of bulk metallic glass—A technology for MEMS and microstructure fabrication. Microelectromech. Syst. J. 2007, 16, 240–247. [Google Scholar] [CrossRef]
© 2014 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martinez-Duarte, R. SU-8 Photolithography as a Toolbox for Carbon MEMS. Micromachines 2014, 5, 766-782. https://doi.org/10.3390/mi5030766
Martinez-Duarte R. SU-8 Photolithography as a Toolbox for Carbon MEMS. Micromachines. 2014; 5(3):766-782. https://doi.org/10.3390/mi5030766
Chicago/Turabian StyleMartinez-Duarte, Rodrigo. 2014. "SU-8 Photolithography as a Toolbox for Carbon MEMS" Micromachines 5, no. 3: 766-782. https://doi.org/10.3390/mi5030766