Saccharomonopyrones A–C, New α-Pyrones from a Marine Sediment-Derived Bacterium Saccharomonospora sp. CNQ-490
Abstract
:1. Introduction
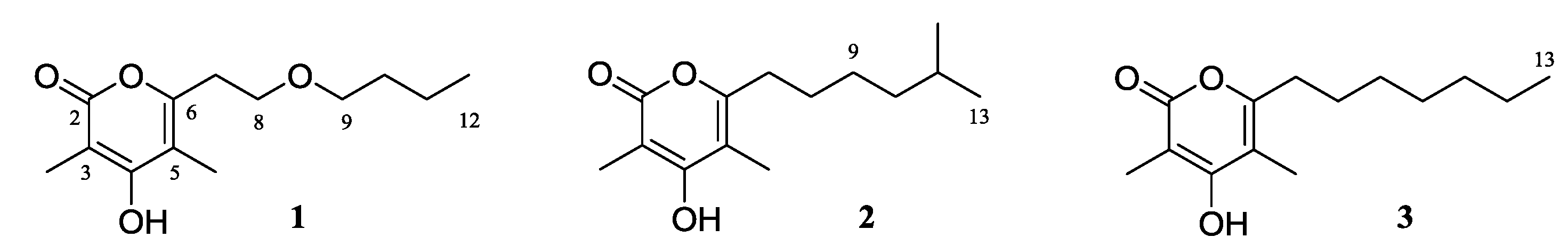
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Strain Isolation and Fermentation
3.3. Extraction and Isolation
3.4. Bioactivity Assays
3.5. Statistical Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Mincer, T.J.; Williams, P.G.; Fenical, W. Marine actinomycete diversity and natural product discovery. Antonie Van Leeuwenhoek 2005, 87, 43–48. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Zheng, L.-Q.; Huang, Y.-J.; Li, W.-J. Saccharomonospora marina sp. nov., isolated from an ocean sediment of the East China Sea. Int. J. Syst. Evol. Microbiol. 2010, 60, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Veyisoglu, A.; Sazak, A.; Cetin, D.; Guven, K.; Sahin, N. Saccharomonospora amisosensis sp. nov., isolated from deep marine sediment. Int. J. Syst. Evol. Microbiol. 2013, 63, 3782–3786. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Lu, M.; Huntemann, M.; Lucas, S.; Lapidus, A.; Copeland, A.; Pitluck, S.; Goodwin, L.A.; Han, C.; Tapia, R.; et al. Genome sequence of the chemoheterotrophic soil bacterium Saccharomonospora cyanea type strain (NA-134(T)). Stand. Genomic Sci. 2013, 9, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Takeda, I. Antibiotic AB-65, a new antibiotic from Saccharomonospora viride. J. Antibiot. 1975, 28, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Parshad, R.; Khajuria, R.K.; Guru, S.K.; Pathania, A.S.; Sharma, R.; Chib, R.; Aravinda, S.; Gupta, V.K.; Khan, I.A.; et al. Saccharonol B, a new cytotoxic methylated isocoumarin from Saccharomonospora azurea. Tetrahedron Lett. 2013, 54, 6695–6699. [Google Scholar] [CrossRef]
- Maloney, K.N.; Macmillan, J.B.; Kauffman, C.A.; Jensen, P.R.; Dipasquale, A.G.; Rheingold, A.L.; Fenical, W. Lodopyridone, a structurally unprecedented alkaloid from a marine actinomycete. Org. Lett. 2009, 11, 5422–5424. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; An, B.-J.; Kim, H.J.; Cho, Y.H.; Kim, D.I.; Jang, J.Y.; Kwak, J.H.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; et al. Anti-Inflammatory Effect of Violapyrones B and C from a Marine-derived Streptomyces sp. Nat. Prod. Sci. 2015, 21, 251–254. [Google Scholar] [CrossRef]
- Shin, H.; Lee, H.-S.; Lee, J.; Shin, J.; Lee, M.; Lee, H.-S.; Lee, Y.-J.; Yun, J.; Kang, J. Violapyrones H and I, New Cytotoxic Compounds Isolated from Streptomyces sp. Associated with the Marine Starfish Acanthaster planci. Mar. Drugs 2014, 12, 3283–3291. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Cao, Y.; Liu, J.; Zheng, D.; Chen, X.; Han, L.; Jiang, C.; Huang, X. Violapyrones A–G, α-pyrone derivatives from Streptomyces violascens isolated from Hylobates hoolock feces. J. Nat. Prod. 2013, 76, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Bertin, M.J.; Demirkiran, O.; Navarro, G.; Moss, N.A.; Lee, J.; Goldgof, G.M.; Vigil, E.; Winzeler, E.A.; Valeriote, F.A.; Gerwick, W.H. Kalkipyrone B, a marine cyanobacterial γ-pyrone possessing cytotoxic and anti-fungal activities. Phytochemistry 2016, 122, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Mierzwa, R.; Xu, L.; He, L.; Terracciano, J.; Patel, M.; Zhao, W.; Black, T.A.; Chan, T.-M. Structure of Sch 419560, a novel α-pyrone antibiotic produced by Pseudomonas fluorescens. J. Antibiot. 2002, 55, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Kawazoe, Y.; Iwasaki, A.; Ohno, O.; Suenaga, K.; Uemura, D. Anti-obesity activities of the yoshinone A and the related marine γ-pyrone compounds. J. Antibiot. 2016, 69, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Inuzuka, T.; Yamamoto, K.; Iwasaki, A.; Ohno, O.; Suenaga, K.; Kawazoe, Y.; Uemura, D. An inhibitor of the adipogenic differentiation of 3T3-L1 cells, yoshinone A, and its analogs, isolated from the marine cyanobacterium Leptolyngbya sp. Tetrahedron Lett. 2014, 55, 6711–6714. [Google Scholar] [CrossRef]
- Brachmann, A.O.; Brameyer, S.; Kresovic, D.; Hitkova, I.; Kopp, Y.; Manske, C.; Schubert, K.; Bode, H.B.; Heermann, R. Pyrones as bacterial signaling molecules. Nat. Chem. Biol. 2013, 9, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.E.; Bassler, B.L. Chemical communication among bacteria. Proc. Natl. Acad. Sci. USA 2003, 100, 14549–14554. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L.; Losick, R. Bacterially speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L. Small Talk. Cell 2002, 109, 421–424. [Google Scholar] [CrossRef]
- Huitt-Roehl, C.R.; Hill, E.A.; Adams, M.M.; Vagstad, A.L.; Li, J.W.; Townsend, C.A. Starter unit flexibility for engineered product synthesis by the nonreducing polyketide synthase PksA. ACS Chem. Biol. 2015, 10, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-F.; Yu, R.-J.; Li, X.-X.; Gao, H.; Guo, L.-D.; Tang, J.-S.; Yao, X.-S. 1H and 13C NMR spectral assignments of 2-pyrone derivatives from an endophytic fungus of sarcosomataceae. Magn. Reson. Chem. 2015, 53, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.L.; Money, T. Pyrone studies. Linear α-pyrone route to protected β-polyketones. Can. J. Chem. 1968, 46, 695–700. [Google Scholar] [CrossRef]
- Kim, Y.; Ogura, H.; Akasaka, K.; Oikawa, T.; Matsuura, N.; Imada, C.; Yasuda, H.; Igarashi, Y. Nocapyrones: α- and γ-pyrones from a marine-derived Nocardiopsis sp. Mar. Drugs 2014, 12, 4110–4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonomura, H.; Ohara, Y. Distribution of actinomycetes in soil. X. New genus and species of monosporic actinomycetes. J. Ferment. Techonol. 1971, 49, 895–903. [Google Scholar]
- Le, T.C.; Yim, C.-Y.; Park, S.; Katila, N.; Yang, I.; Song, M.C.; Yoon, Y.J.; Choi, D.-Y.; Choi, H.; Nam, S.-J.; et al. Lodopyridones B and C from a marine sediment-derived bacterium Saccharomonospora sp. Bioorg. Med. Chem. Lett. 2017, 27, 3123–3126, in press. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Reynolds, K.A.; Kersten, R.D.; Ryan, K.S.; Gonzalez, D.J.; Nizet, V.; Dorrestein, P.C.; Moore, B.S. Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc. Natl. Acad. Sci. USA 2014, 111, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Koryakina, I.; McArthur, J.B.; Draelos, M.M.; Williams, G.J. Promiscuity of a modular polyketide synthase towards natural and non-natural extender units. Org. Biomol. Chem. 2013, 11, 4449–4458. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Choi, H.; Nam, S.-J.; Fenical, W.; Kim, H. Potent Inhibition of Monoamine Oxidase B by a Piloquinone from Marine-Derived Streptomyces sp. CNQ-027. J. Microbiol. Biotechnol. 2017, 27, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, I.; Patil, R.S.; Kang, S.; Lee, J.; Choi, H.; Kim, M.-S.; Nam, S.-J.; Kang, H. Anithiactins A-C, modified 2-phenylthiazoles from a mudflat-derived Streptomyces sp. J. Nat. Prod. 2014, 77, 2716–2719. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, K.-J.; Yeon, J.-T.; Kim, S.H.; Won, D.H.; Choi, H.; Nam, S.-J.; Son, Y.-J.; Kang, H. Placotylene A, an inhibitor of the receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation, from a Korean sponge Placospongia sp. Mar. Drugs 2014, 12, 2054–2065. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Leutou, A.S.; Jeong, H.; Kim, D.; Seong, C.N.; Nam, S.-J.; Lim, K.-M. Anti-pigmentary effect of (−)-4-hydroxysattabacin from the marine-derived bacterium Bacillus sp. Mar. Drugs 2017, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Leutou, A.S.; Yang, I.; Kang, H.; Seo, E.K.; Nam, S.-J.; Fenical, W. Nocarimidazoles A and B from a Marine-Derived Actinomycete of the Genus Nocardiopsis. J. Nat. Prod. 2015, 78, 2846–2849. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.H.; Priyadarshini, G.; Michael, T. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar]
- Roberta, R.; Nicoletta, P.; Anna, P.; Ananth, P.; Min, Y.; Catherine, R.E. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]

| No. C | (1) | (2) | (3) | |||
|---|---|---|---|---|---|---|
| δC, Type | δH, Mult. 2 (J in Hz) | δC, Type | δH, Mult. 2 (J in Hz) | δC, Type | δH, Mult. 2 (J in Hz) | |
| 2 | 164.5 | 165.1 | 164.6 | |||
| 3 | 97.3 | 97.6 | 97.1 | |||
| 4 | 164.3 | 164.9 | 164.4 | |||
| 5 | 107.5 | 107.0 | 106.5 | |||
| 6 | 155.2 | 158.2 | 157.8 | |||
| 7 | 31.0, CH2 | 2.70, t (6.4) | 30.4, CH2 | 2.47, t (6.5) | 29.9, CH2 | 2.45, t (7.5) |
| 8 | 67.0, CH2 | 3.56. t (6.4) | 27.6, CH2 | 1.51, m 3 | 26.8, CH2 | 1.51, m |
| 9 | 69.6, CH2 | 3.35, t (6.4) | 26.6, CH2 | 1.29, m | 28.3, CH2 | 1.26, m 3 |
| 10 | 31.1, CH2 | 1.43, m | 38.4, CH2 | 1.17, m | 28.3, CH2 | 1.26, m 3 |
| 11 | 18.7, CH2 | 1.26, m | 27.8, CH | 1.51, m3 | 31.1, CH2 | 1.25, m 3 |
| 12 | 13.6, CH3 | 0.83, t (7.2) | 22.9, CH3 | 0.85, d (6.6) | 22.0, CH2 | 1.26, m 3 |
| 13 | 22.9, CH3 | 0.85, d (6.6) | 13.8, CH3 | 0.85, t (7.1) | ||
| 3-CH3 | 9.1, CH3 | 1.82, s | 9.6, CH3 | 1.83, s | 9.1, CH3 | 1.82, s |
| 5-CH3 | 9.9, CH3 | 1.87, s | 10.3, CH3 | 1.88, s | 9.8, CH3 | 1.87, s |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yim, C.-Y.; Le, T.C.; Lee, T.G.; Yang, I.; Choi, H.; Lee, J.; Kang, K.-Y.; Lee, J.S.; Lim, K.-M.; Yee, S.-T.; et al. Saccharomonopyrones A–C, New α-Pyrones from a Marine Sediment-Derived Bacterium Saccharomonospora sp. CNQ-490. Mar. Drugs 2017, 15, 239. https://doi.org/10.3390/md15080239
Yim C-Y, Le TC, Lee TG, Yang I, Choi H, Lee J, Kang K-Y, Lee JS, Lim K-M, Yee S-T, et al. Saccharomonopyrones A–C, New α-Pyrones from a Marine Sediment-Derived Bacterium Saccharomonospora sp. CNQ-490. Marine Drugs. 2017; 15(8):239. https://doi.org/10.3390/md15080239
Chicago/Turabian StyleYim, Chae-Yoon, Tu Cam Le, Tae Gu Lee, Inho Yang, Hansol Choi, Jusung Lee, Kyung-Yun Kang, Jin Sil Lee, Kyung-Min Lim, Sung-Tae Yee, and et al. 2017. "Saccharomonopyrones A–C, New α-Pyrones from a Marine Sediment-Derived Bacterium Saccharomonospora sp. CNQ-490" Marine Drugs 15, no. 8: 239. https://doi.org/10.3390/md15080239





