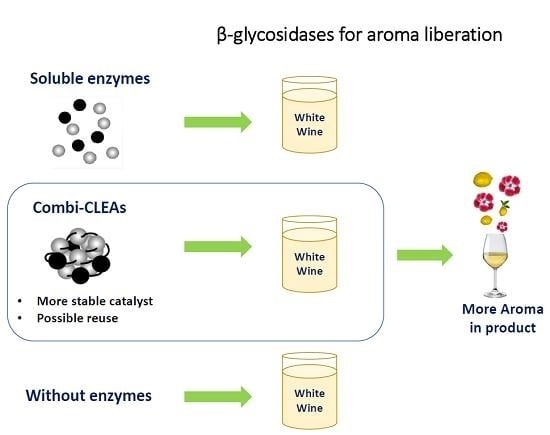
Aroma Release in Wine Using Co-Immobilized Enzyme Aggregates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Combi-CLEAs
2.2. Stability of Combi-CLEAs
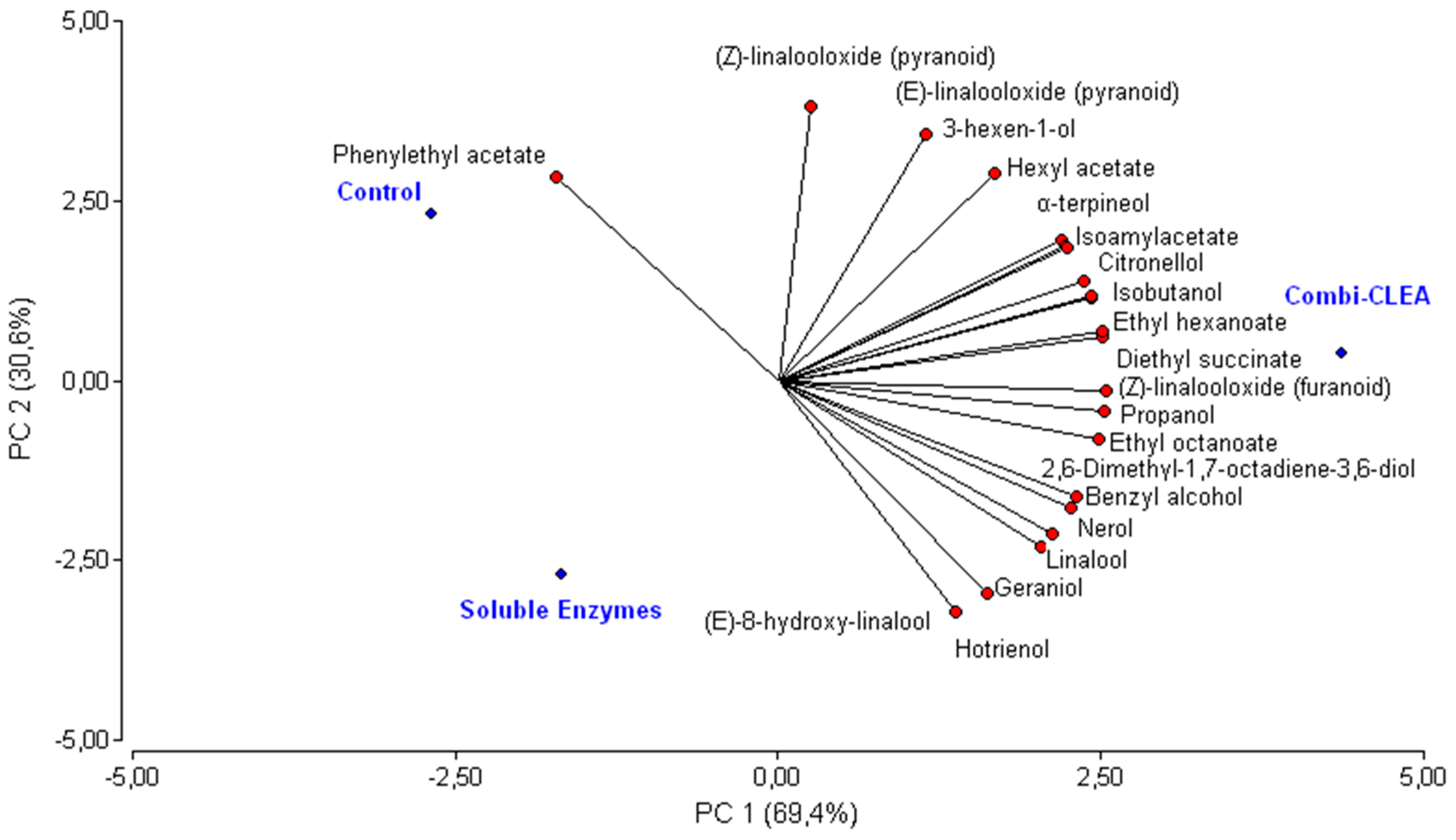
2.3. Volatile Compounds in Muscat Wines
2.4. Treatments Classification
3. Materials and Methods
3.1. Materials
3.2. Hydrolytic Activity
3.3. Preparation of Combi-CLEAs
3.4. Stability of Combi-CLEAs
3.5. Winemaking
3.6. Enzymatic Treatment of Wine
3.7. Oenological Parameter Analysis
3.8. Analysis of Volatile Compounds by GC-MS
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baumes, R.L.; Aubert, C.C.; Günata, Z.Y.; De Moor, W.; Bayonove, C.L.; Tapiero, C. Structures of two C13-norisoprenoid glucosidic precursors of wine flavor. J. Essent. Oil Res. 1994, 6, 587–599. [Google Scholar] [CrossRef]
- Günata, Z.; Bitteur, S.; Brillouet, J.; Bayonove, C.; Cordonnier, R. Sequential enzymic hydrolysis of potentially aromatic glycosides from grape. Carbohydr. Res. 1988, 184, 139–149. [Google Scholar] [CrossRef]
- Günata, Z.; Bayonove, C.; Tapiero, C.; Cordonnier, R. Hydrolysis of grape monoterpenyl β-d-glucosides by various β-glucosidases. J. Agric. Food Chem. 1990, 38, 1232–1236. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Betancor, L.; Luckarift, H.R. Co-immobilized coupled enzyme systems in biotechnology. Biotechnol. Genet. Eng. 2010, 27, 95–114. [Google Scholar] [CrossRef]
- Guisán, J.M. Aldehyde-agarose gels as activated supports for immobilization-stabilization of enzymes. Enzyme Microb. Technol. 1998, 10, 375–382. [Google Scholar] [CrossRef]
- Gupta, M.N. Thermostabilization of proteins. Biotechnol. Appl. Biochem. 1991, 4, 1–11. [Google Scholar]
- Mateo, C.; Abián, O.; Fernández-Lafuente, R.; Guisán, J.M. Increase in conformational stability of enzymes immobilized on epoxy-activated supports by favoring additional multipoint covalent attachment. Enzyme Microb. Technol. 2000, 26, 509–515. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef]
- Peirce, S.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Rueda, N.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Russo, M.E.; Marzocchella, A.; Fernandez-Lafuente, R. Development of simple protocols to solve the problems of enzyme coimmobilization. Application to coimmobilize a lipase and a β-galactosidase. RSC Adv. 2016, 6, 61707–61715. [Google Scholar] [CrossRef]
- Kazenwadel, F.; Franzreb, M.; Rapp, B.E. Synthetic enzyme supercomplexes: Co-immobilization of enzyme cascades. Anal. Methods 2015, 7, 4030–4037. [Google Scholar] [CrossRef]
- Sheldon, R. Enzyme immobilization: the quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Talekar, S.; Desai, S.; Pillai, M.; Nagavekar, N.; Ambarkar, S.; Surnis, S.; Ladole, M.; Nadar, S.; Mulla, M. Carrier free co-inmobilization of glucoamylase and pullulanase as combi-cross linked enzyme aggregates (combi-CLEAs). RSC Adv. 2013, 3, 2265–2271. [Google Scholar] [CrossRef]
- Dalal, S.; Kapoor, M.; Gupta, M.N. Preparation and characterization of combi-CLEAs catalyzing multiple non-cascade reactions. J. Mol. Catal. B Enzym. 2007, 44, 128–132. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G. Progress in co-immobilization of multiple enzymes. Shengwu Gongcheng Xuebao/Chin. J. Biotechnol. 2015, 31, 469–480. [Google Scholar]
- Nouaimi-Bachmann, M.; Skilewitsch, O.; Senhaji-Dachtler, S.; Bisswanger, H. Co-immobilization of different enzyme activities to non-woven polyester surfaces. Biotechnol. Bioeng. 2007, 96, 623–630. [Google Scholar] [CrossRef]
- Betancor, L.; Berne, C.; Luckarift, H.R.; Spain, J.C. Coimmobilization of a redox enzyme and a cofactor regeneration system. Chem. Commun. 2006, 34, 3640–3642. [Google Scholar] [CrossRef]
- Ahumada, K.; Urrutia, P.; Illanes, A.; Wilson, L. Production of combi-CLEAs of glycosidases utilized for aroma enhancement in wine. Food Bioprod. Process. 2015, 94, 555–560. [Google Scholar] [CrossRef]
- González-Pombo, P.; Fariña, L.; Carrau, F.; Batista-Viera, F.; Brena, B. Aroma enhancement in wines using co-immobilized Aspergillus. niger glycosidases. Food Chem. 2014, 143, 185–191. [Google Scholar] [CrossRef]
- Ebeler, S.E.; Thorngate, J.H. Wine chemistry and flavor: Looking into the crystal glass. J. Agric. Food Chem. 2009, 57, 8098–8108. [Google Scholar] [CrossRef]
- Mateo, J.; Jimenez, M. Monoterpenes in grape juice and wines. Review. J. Chromatogr. A. 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Belancic, A.; Agosin, E.; Ibacache, A.; Bordeau, E.; Baumes, R.; Razungles, A.; Bayonove, C. Influence of sun exposure on the aromatic composition of Chilean Muscat grape cultivars Moscatel de Alejandria and Moscatel rosada. Am. J. Enol. Vitic. 1997, 482, 181–186. [Google Scholar]
- Bayonove, C.; Cordonnier, R. Récherches sur l’arôme du muscat. III Étude de la fraction terpénique. Ann. Technol. Agric. 1971, 20, 347–355. [Google Scholar]
- Günata, Y.Z.; Bayonove, C.; Baumes, R.; Cordonnier, R. The aroma of grapes. I. Extraction and determination of free and glycosidically bound fraction of some grape aroma components. J. Chromatogr. A. 1985, 331, 83–90. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Masa, A.; Oliveira, J.M. Correlation between volatile composition and sensory properties in Spanish Albariño wines. Microchem. J. 2010, 95, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.; López, R.; Caho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 85, 1659–1667. [Google Scholar] [CrossRef]
- Romo-Sánchez, S.; Arévalo-Villena, M.; García Romero, E.; Ramirez, H.L.; Briones Pérez, A. Immobilization of β-glucosidase and its application for enhancement of aroma precursors in muscat wine. Food Bioprocess Technol. 2013, 7, 1381–1392. [Google Scholar] [CrossRef]
- Marias, J. Terpenes in the aroma of grapes and wines: A review. S Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar]
- Sefton, M.A.; Francis, I.L.; Williams, P.J. The volatile composition of Chardonnay juices: A study by flavour precursor analysis. Am. J. Enol. Vitic. 1993, 44, 359–370. [Google Scholar]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Aroma potential of Brancellao grapes from different cluster positions. Food Chem. 2012, 132, 112–124. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Díaz-Maroto Hidalgo, M.C.; González-Viñas, M.Á.; Pérez-Coello, M.S. Aroma enhancement in wines from different grape varieties using exogenous glycosidases. Food Chem. 2005, 92, 627–635. [Google Scholar] [CrossRef]
- Fariña, L.; Villar, V.; Aresc, G.; Carrau, F.; Dellacassad, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar]
- Jiang, B.; Zhang, Z. Volatile compounds of young wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay varieties grown in the Loess Plateau Region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Henley, J.P.; Sadana, A. Categorization of enzyme deactivations using a series-type mechanism. Enzyme Microb. Technol. 1985, 7, 50–60. [Google Scholar] [CrossRef]
- Henley, J.P.; Sadana, A. Deactivation theory. Biotechnol. Bioeng. 1986, 28, 1277–1285. [Google Scholar] [CrossRef]
- OIV. Methods of analysis of wines and musts. In Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2003; Volume 1, pp. 16–31. [Google Scholar]
- López, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the combi-CLEAs are available from the authors.

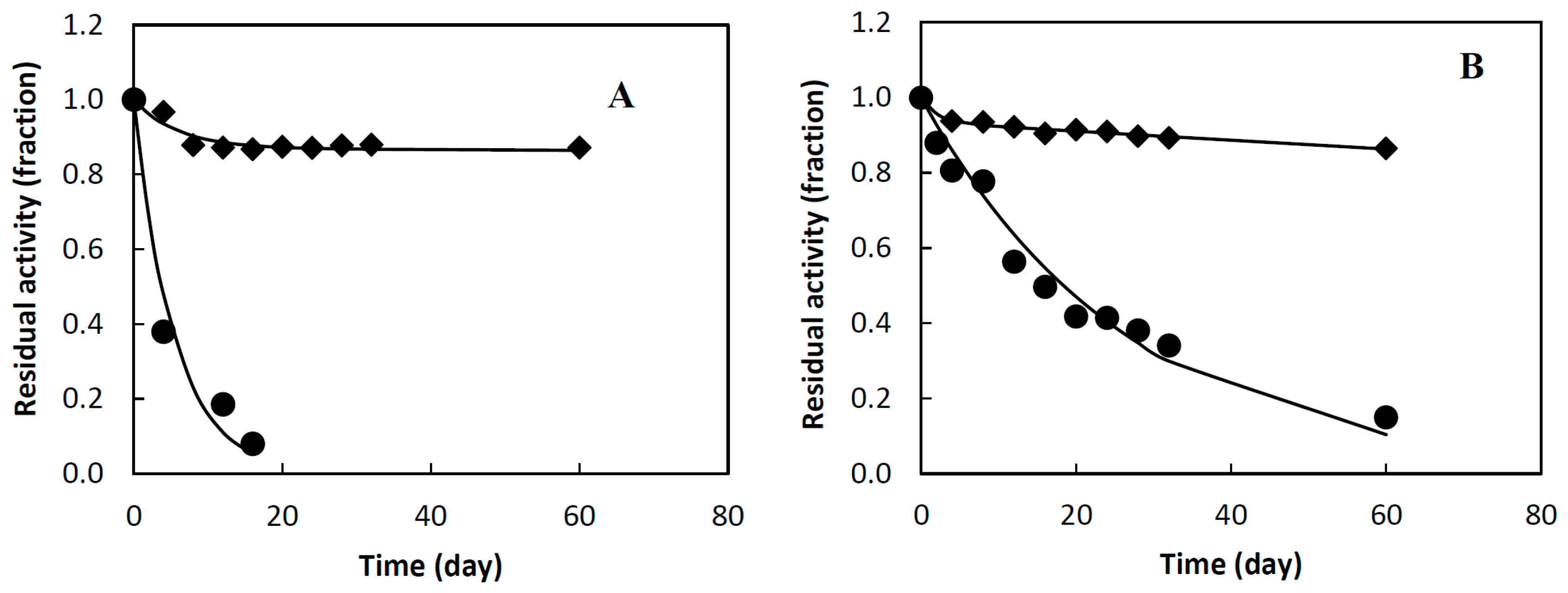

| Biocatalyts | Parameters | |||||
|---|---|---|---|---|---|---|
| a | k1 (d−1) | k2 (d−1) | R2 | t1/2 (d) | SF | |
| βG soluble | 0.18 | 0.94 | 3.8 | |||
| βG in combi-CLEA | 0.87 | 0.17 | 0.0001 | 0.85 | 5537 | 1460 |
| ARA soluble | 0.04 | 0.95 | 18.4 | |||
| ARA in combi-CLEA | 0.93 | 0.48 | 0.00134 | 0.97 | 15510 | 843 |
| Compounds | Odor Threshold (Reference) | Control | Soluble Enzymes | Combi-CLEA |
|---|---|---|---|---|
| Terpenes µg/L | ||||
| Linalool | 25 [27] | 415.98 ± 0.98 a | 469.62 ± 32.14 ab | 495.83 ± 43.26 b |
| Citronellol | 100 [27] | 67.23 ± 1.40 ab | 65.75 ± 2.61 b | 71.74 ± 3.11 b |
| Nerol | 400 [27] | 270.59 ± 8.81 a | 300.64 ± 2.36 b | 322.81 ± 10.40 c |
| Geraniol | 30 [28] | 265.58 ± 4.30 a | 499.32 ± 47.24 b | 587.58 ± 11.52 c |
| α-Terpineol | 250 [27] | 177.89 ± 4.22 b | 165.92 ± 2.80 a | 194.71 ± 4.72 c |
| (Z)-Linalooloxide (furanoid) | 6000 [34] | 18.33 ± 0.34 a | 18.27 ± 0.1.29 a | 20.65 ± 0.14 b |
| Hotrienol | 110 [30] | 19.40 ± 1.03 a | 26.93 ± 4.49 b | 27.20 ± 0.21 b |
| (E)-Linalooloxide (pyranoid) | 3000 [34] | 144.99 ± 9.00 b | 126.6 ± 9.22 a | 146.61 ± 0.19 b |
| (Z)-Linalooloxide (pyranoid) | 3000 [34] | 61.26 ± 0.03 a | 55.15 ± 5.43 a | 59.45 ± 0.06 a |
| 2.6-Dimethyl-1.7-octadiene-3.6-diol | 143.25 ± 2.83 a | 151.22 ± 16.05 ab | 166.41 ± 3.15 b | |
| (E)-8-Hydroxylinalool (trans-2.6-dimethyl-2.7-octadien-1.6-diol) | 27.05 ± 2.12 a | 124.17 ± 5.94 b | 115.21 ± 5.65 b | |
| Total | 2526.62 a | 2900.75 b | 2999.01 b | |
| Alcohols µg/L | ||||
| Propanol | 750,000 [27] | 22,141.24 ± 2293.86 a | 22,598.11 ± 1577.10 a | 24,665.39 ± 952.10 a |
| Isobutanol | 40,000 [28] | 33,865.40 ± 228.71 a | 33,506.26 ± 3617.49 a | 35,440.50 ± 3970.30 a |
| Benzyl alcohol | 200,000 [35] | 155.98 ± 6.42 a | 219.44 ± 0.93 b | 274.53 ± 48.76 b |
| cis-3-Hexen-1-ol | 400 [27] | 154.48 ± 8.58 ab | 135.64 ± 3.16 a | 163.75 ± 18.55 b |
| Total | 56317.09 a | 56459.46 a | 60544.17 a | |
| Esters µg/L | ||||
| Isoamylacetate | 30 [35] | 7725.37 ± 264.72 a | 7505.14 ± 45.35 a | 8090.17 ± 563.83 a |
| Hexyl acetate | 670 [35] | 380.17 ± 17.74 a | 364.70 ± 14.25 a | 406.99 ± 3.86 b |
| Phenylethyl acetate | 250 [27] | 1722.54 ± 1.97 b | 1579.22 ± 46.55 a | 1564.80 ± 50.11 a |
| Ethyl hexanoate | 14 [32] | 960.09 ± 15.49 a | 923.50 ± 46.01 a | 1117.61 ± 14.39 b |
| Ethyl octanoate | 5 [32] | 1589.86 ± 45.16 a | 1689.92 ± 99.26 a | 1985.06 ± 51.83 b |
| Diethyl succinate | 500,000 [32] | 1019.75 ± 84.30 a | 1005.84 ± 75.63 a | 1254.76 ± 24.37 b |
| Total | 13,397.78 ab | 13,068.32 a | 14,419.4 b |
| Treatment | pH | Total Acidity (g/L) a | Volatile Acidity (g /L) b | Alcohol (v/v %) |
|---|---|---|---|---|
| Control | 3.97 | 3.67 | 0.32 | 13.0 |
| Soluble enzymes | 3.99 | 3.53 | 0.38 | 13.0 |
| Combi-CLEA | 3.98 | 3.63 | 0.34 | 13.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahumada, K.; Martínez-Gil, A.; Moreno-Simunovic, Y.; Illanes, A.; Wilson, L. Aroma Release in Wine Using Co-Immobilized Enzyme Aggregates. Molecules 2016, 21, 1485. https://doi.org/10.3390/molecules21111485
Ahumada K, Martínez-Gil A, Moreno-Simunovic Y, Illanes A, Wilson L. Aroma Release in Wine Using Co-Immobilized Enzyme Aggregates. Molecules. 2016; 21(11):1485. https://doi.org/10.3390/molecules21111485
Chicago/Turabian StyleAhumada, Katherine, Ana Martínez-Gil, Yerko Moreno-Simunovic, Andrés Illanes, and Lorena Wilson. 2016. "Aroma Release in Wine Using Co-Immobilized Enzyme Aggregates" Molecules 21, no. 11: 1485. https://doi.org/10.3390/molecules21111485