Synthesis and In Vitro Anti Leishmania amazonensis Biological Screening of Morita-Baylis-Hillman Adducts Prepared from Eugenol, Thymol and Carvacrol
Abstract
:1. Introduction
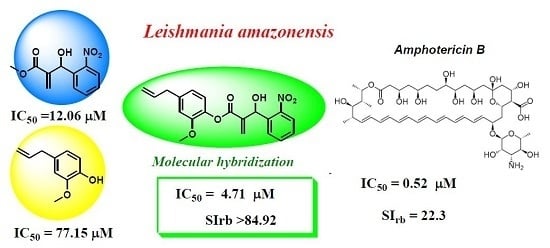
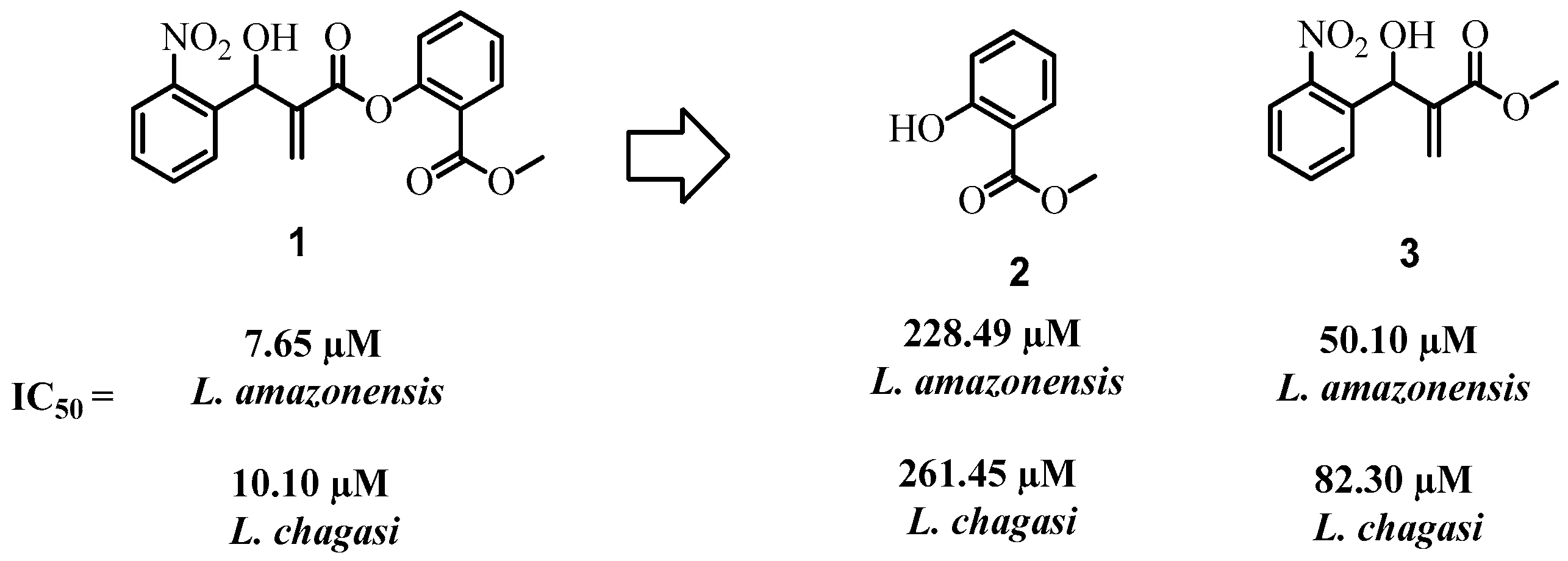
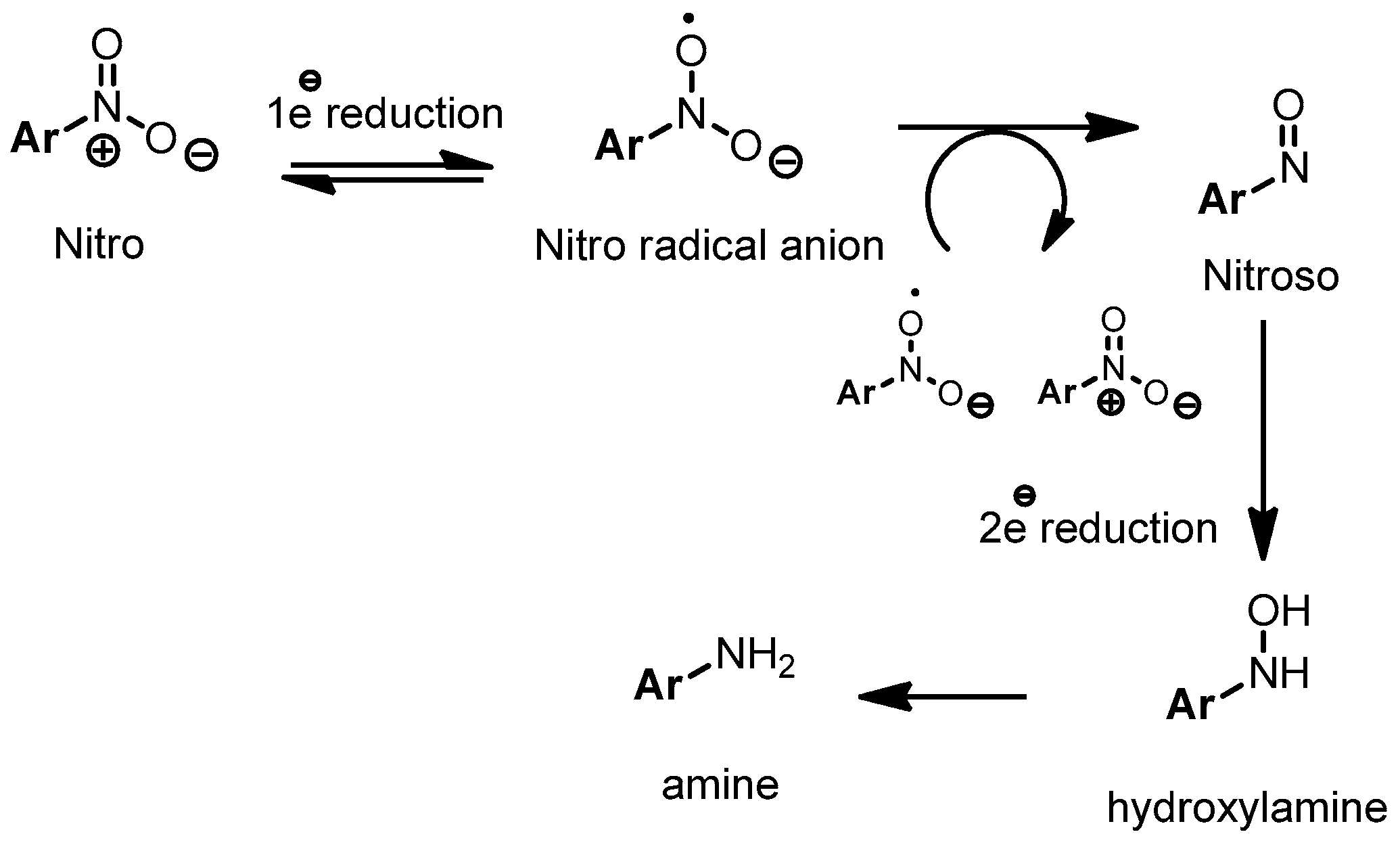
2. Results and Discussion
3. Materials and Methods
3.1. Experimental Chemistry
General
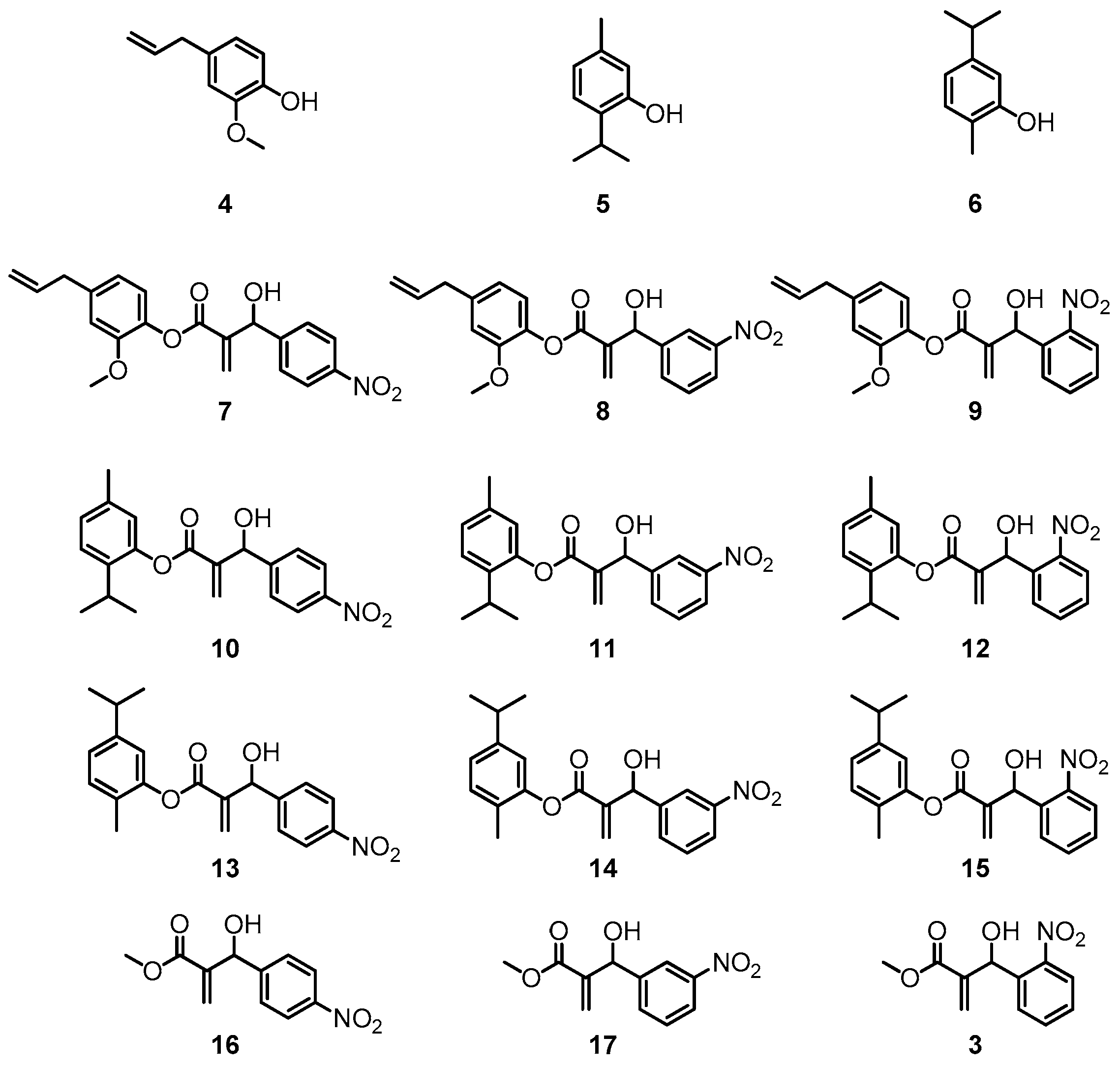
3.2. General Procedure for Esterification of Eugenol (4), Thymol (5) and Carvacrol (6) with Acrylic Acid; Preparation of Compounds 18–20
3.2.1. 4-Allyl-2-methoxyphenyl acrylate (18)
3.2.2. 2-Isopropyl-5-methylphenyl acrylate (19)
3.2.3. 5-Isopropyl-2-methylphenyl acrylate (20)
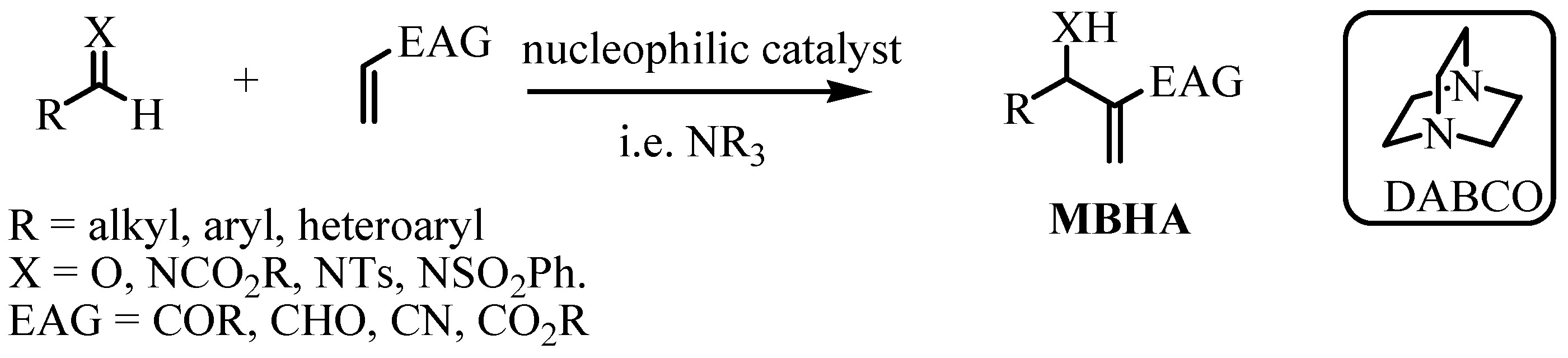
3.3. General Synthesis of the MBHAs 16, 17 and 3
3.3.1. 2-[Hydroxy(4-nitrophenyl)methyl]propanoate (16)
3.3.2. 2-[Hydroxy(3-nitrophenyl)methyl]propanoate (17)
3.3.3. 2-[Hydroxy(2-nitrophenyl)methyl]propanoate (3)
3.4. General Synthesis of the Hybrids 7–15
3.4.1. 4-Allyl-2-methoxyphenyl 2-(hydroxy(4-nitrophenyl)methyl)acrylate (7)
3.4.2. 4-Allyl-2-methoxyphenyl-2-(hydroxy(3-nitrophenyl)methyl)acrylate (8)
3.4.3. 4-Allyl-2-methoxyphenyl 2-(hydroxy(2-nitrophenyl)methyl)acrylate (9)
3.4.4. 2-Isopropyl-5-methylphenyl 2-(hydroxy(4-nitrophenyl)methyl)acrylate (10)
3.4.5. 2-Isopropyl-5-methylphenyl 2-(hydroxy(3-nitrophenyl)methyl)acrylate (11)
3.4.6. 2-Isopropyl-5-methylphenyl 2-(hydroxy(2-nitrophenyl)methyl)acrylate (12)
3.4.7. 5-Isopropyl-2-methylphenyl 2-(hydroxy(4-nitrophenyl)methyl)acrylate (13)
3.4.8. 5-Isopropyl-2-methylphenyl 2-(hydroxy(3-nitrophenyl)methyl)acrylate (14)
3.4.9. 5-Isopropyl-2-methylphenyl 2-(hydroxy(2-nitrophenyl)methyl)acrylate (15)
3.5. Biology
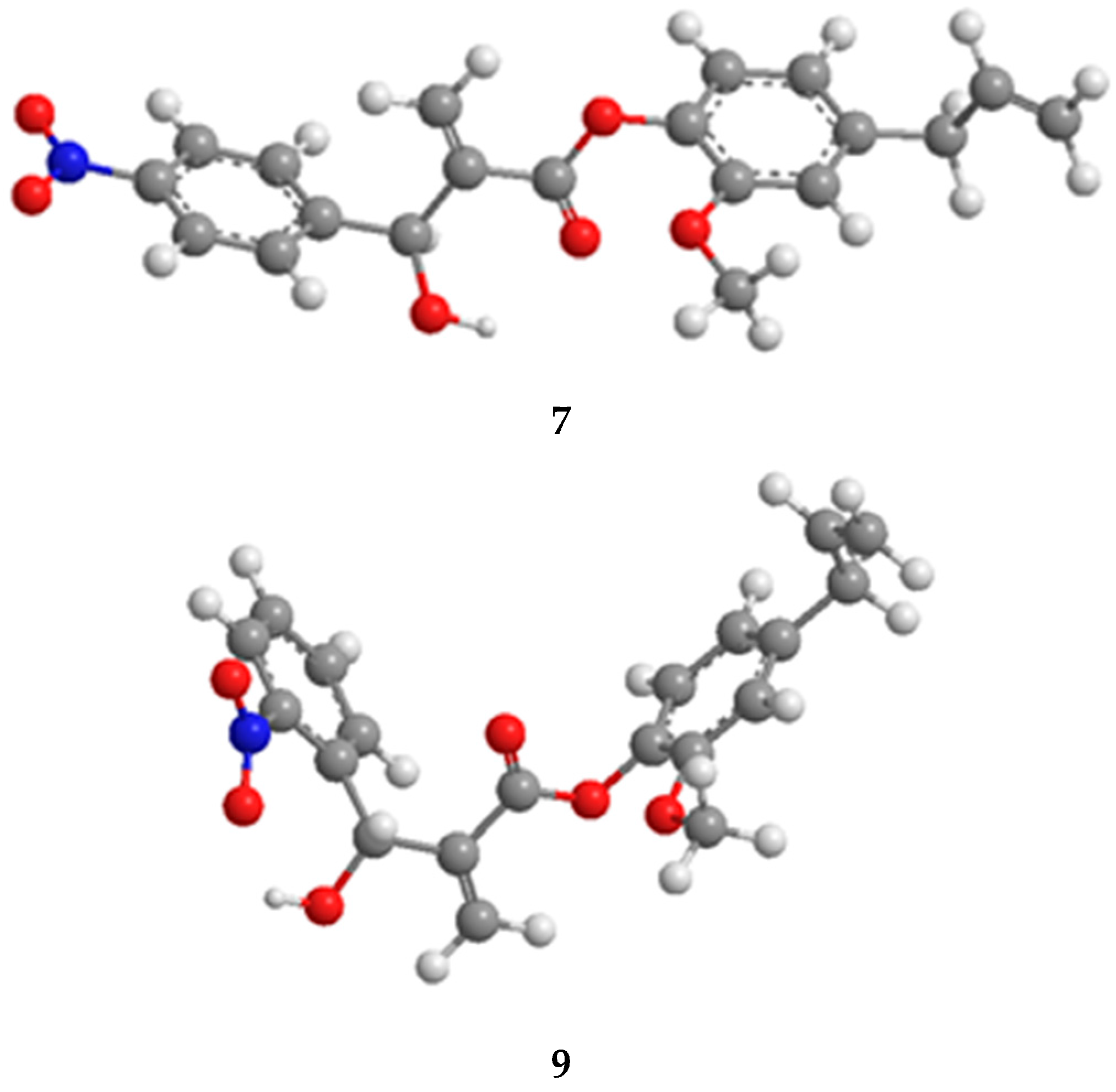
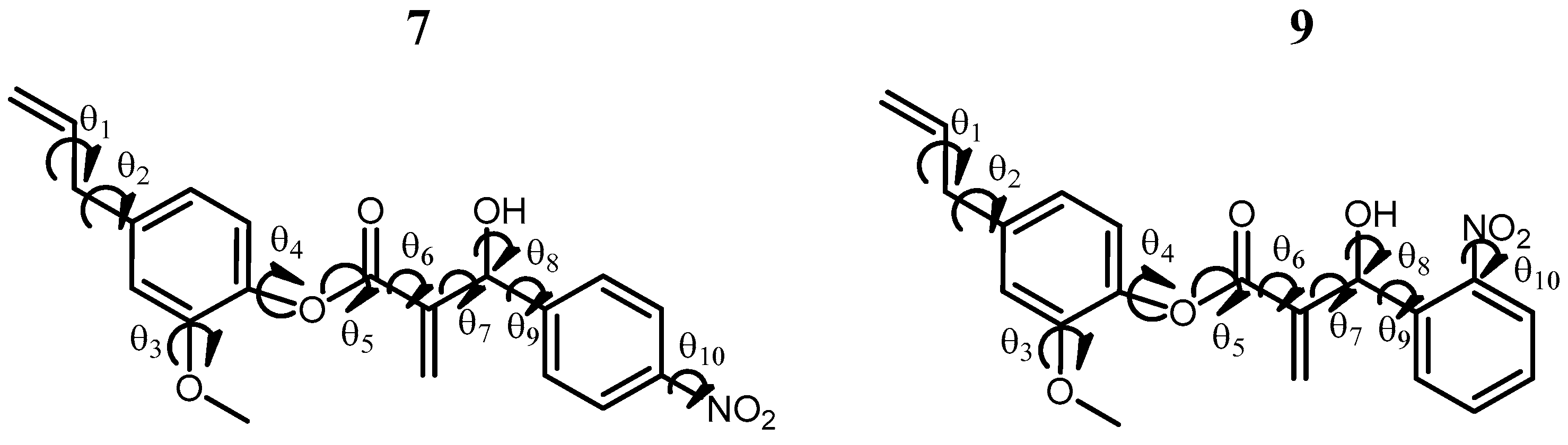
3.6. Conformational Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author contributions
Conflicts of Interest
References
- Alvar, J; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den-Boer, M. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Volpinia, Â.C.; Passos, V.M.A.; Oliveira, G.C.; Romanha, A.J. PCR-RFLP to identify Leishmania (Viannia) braziliensis and L. (Leishmania) amazonensis causing American cutaneous leishmaniasis. Acta Tropica 2004, 90, 31–37. [Google Scholar] [CrossRef]
- Leishmaniasis. Available online: http://www.who.int/leishmaniasis/en/ (accessed on 10 March 2016).
- Rafik, L.; Rhaiem, B.; Houimel, M. Targeting Leishmania major parasite with peptides derived from a combinatorial phage display library. Acta Tropica 2016, 159, 11–19. [Google Scholar]
- Croft, S.L.; Coombs, G.H. Leishmaniasis-current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003, 19, 502–508. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Khan, F.A.K.; Kulkarni, A.A.; Arote, R.; Patil, R.H. Antileishmanial drug discovery: Comprehensive review of the last 10 years. RSC Adv. 2015, 5, 32376–32415. [Google Scholar] [CrossRef]
- Lima-Junior, C.G.; Vasconcellos, M.L.A.A. Morita-Baylis-Hillman adducts: Biological activities and potentialities to the discovery of new cheaper drugs. Bioorg. Med. Chem. 2012, 20, 3954–3971. [Google Scholar] [CrossRef]
- Basavaiah, D.; Reddy, B.S.; Badsara, S.S. Recent Contributions from the Baylis−Hillman Reaction to Organic Chemistry. Chem. Rev. 2010, 110, 5447–5674. [Google Scholar] [CrossRef]
- Santos, M.S.; Coelho, F.; Lima-Junior, C.G.; Vasconcellos, M.L.A.A. The Morita-Baylis-Hillman Reaction: Advances and Contributions from Brazilian Chemistry. Curr. Org. Synth. 2015, 12, 830–852. [Google Scholar] [CrossRef]
- Barbosa, T.P.; Sousa, S.C.O.; Amorim, F.M.; Rodrigues, Y.K.S.; De Assis, P.A.C.; Caldas, J.P.A.; Oliveira, M.R.; Vasconcellos, M.L.A.A. Design, synthesis and antileishmanial in vitro activity of new series of chalcones-like compounds: A molecular hybridization approach. Bioorg. Med. Chem. 2011, 19, 4250–4256. [Google Scholar] [CrossRef]
- De Souza, R.O.M.A.; Pereira, V.L.P.; Muzitano, M.F.; Rossi-Bergmann, B.; Filho, E.B.A.; Vasconcellos, M.L.A.A. High selective leishmanicidal activity of 3-hydroxy-2-methylene-3-(4-bromophenyl) propanenitrile and analogous compounds. Eur. J. Med. Chem. 2007, 42, 99–102. [Google Scholar] [CrossRef]
- Barbosa, T.C.; Lima, C.G., Jr.; Silva, F.P.L.; Lopes, H.M.; Figueiredo, L.R.F.; Souza, S.C.O.; Batista, G.N.; Silva, T.G.; Silva, T.M.; Oliveira, M.R.; et al. Improved synthesis of seven aromatic Baylis-Hillman adducts (BHA): Evaluation against Artemia salina Leach. and Leishmania chagasi. Eur. J. Med. Chem. 2009, 44, 1726–1730. [Google Scholar] [CrossRef]
- Junior, C.G.L.; de Assis, P.A.C.; Silva, F.P.L.; Sousa, S.C.O.; de Andrade, N.G.; Barbosa, T.P.; Nerís, P.L.N.; Segundo, L.V.G.; Anjos, I.C.; Carvalho, G.A.U.; et al. Efficient synthesis of 16 aromatic Morita–Baylis–Hillman adducts: Biological evaluation on Leishmania amazonensis and Leishmania chagasi. Bioorg. Chem. 2010, 38, 279–284. [Google Scholar] [CrossRef]
- Silva, F.P.L.; de Assis, P.A.C.; Lima-Junior, C.G.; de Andrade, N.G.; da Cunha, S.M.D.; Oliveira, M.R.; Vasconcellos, M.L.A.A. Synthesis, evaluation against Leishmania amazonensis and cytotoxicity assays in macrophages of sixteen new congeners Morita-Baylis-Hillman adducts. Eur. J. Med. Chem. 2011, 46, 4295–4301. [Google Scholar] [CrossRef]
- Da Silva, W.A.V.; Rodrigues, D.C.; Oliveira, R.G.; Mendes, R.K.S.; Olegário, T.R.; Rocha, J.C.; Keesen, T.S.L.; Lima, C.G., Jr.; Vasconcellos, M.L.A.A. Synthesis and activity of novel homodimers of Morita-Baylis-Hillman adducts against Leishmania donovani: A twin drug approach. Bioorg. Med. Chem. Lett. 2016, 26, 4523–4526. [Google Scholar] [CrossRef]
- Islamuddin, M.; Sahal, D.; Afrin, F. Apoptosis-like death in Leishmania donovani promastigotes induced by eugenol-rich oil of Syzygium aromaticum. J. Med. Microbiol. 2014, 63, 74–85. [Google Scholar] [CrossRef]
- Morais, S.M.; Vila-Nova, N.S.; Bevilaqua, C.M.L.; Rondon, F.C.; Lobo, C.H.; Moura, A.A.A.; Sales, N.A.D.; Rodrigues, A.P.R.; Figuereido, J.R.; Campello, C.C.; et al. Thymol and eugenol derivatives as potential antileishmanial agentes. Bioorg. Med. Chem. 2014, 22, 6250–6255. [Google Scholar] [CrossRef]
- Farias, P.A., Jr.; Rios, M.C.; Moura, T.A.; Almeida, R.P.; Alves, P.B.; Blank, A.F.; Fernandes, R.P.; Scher, M.R. Leishmanicidal activity of carvacrol-rich essential oil from Lippia sidoides Cham. Biol. Res. 2012, 45, 399–402. [Google Scholar] [CrossRef]
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef]
- Paiva, Y.G.; Souza, A.A.; Lima, C.G., Jr.; Silva, F.P.L.; Filho, E.B.A.; Vasconcelos, C.C.; Abreu, F.C.; Goulart, M.O.F.; Vasconcellos, M.L.A.A. Correlation between electrochemical and theoretical studies on the leishmanicidal activity of twelve Morita-Baylis-Hillman adducts. J. Braz. Chem. Soc. 2012, 23, 894–904. [Google Scholar] [CrossRef]
- Paiva, Y.G.; Júnior, W.P.A.; de Souza, A.; Costa, C.O.; Silva, F.P.L.; Lima, C.G., Jr.; Vasconcellos, M.L.A.A.; Goulart, M.O.F. Electrochemical and computational studies, in protic medium, of Morita-Baylis-Hillman adducts and correlation with leishmanicidal activity. Electrochim. Acta 2014, 140, 557–563. [Google Scholar] [CrossRef]
- Filho, E.B.A.; Ventura, E.; do Monte, S.A.; Oliveira, B.G.; Junior, C.G.L.; Rocha, G.B.; Vasconcellos, M.L.A.A. Synthesis and conformational study of a new class of highly bioactive compounds. Chem. Phys. Lett. 2007, 449, 336–340. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rodrigues, K.A.F.; Dias, C.N.S.; Néris, P.L.N.; Rocha, J.C.; Scotti, M.T.; Scotti, L.; Mascarenhas, S.R.; Veras, R.C.; de Medeiros, I.A.; Keesen, T.S.L.; et al. 2-Amino-thiophene derivatives present antileishmanial activity mediated by apoptosis and immunomodulation in vitro. Eur. J. Med. Chem. 2015, 106, 1–14. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01. Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Xavier, F.J.S.; Neto, J.S.S.; Néris, P.L.N.; Oliveira, M.R.; Vale, J.A.; Vasconcellos, M.L.A.A. Kinetic resolution of leishmanicidal meta and para (±)-2-[Hydroxy(nitrophenyl) methyl]acrylonitrile catalyzed by CALB: In vitro evaluations of separated meta (R), (S) and (R/S) adducts. J. Mol. Catal. B. Enzyn. 2014, 108, 7–12. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
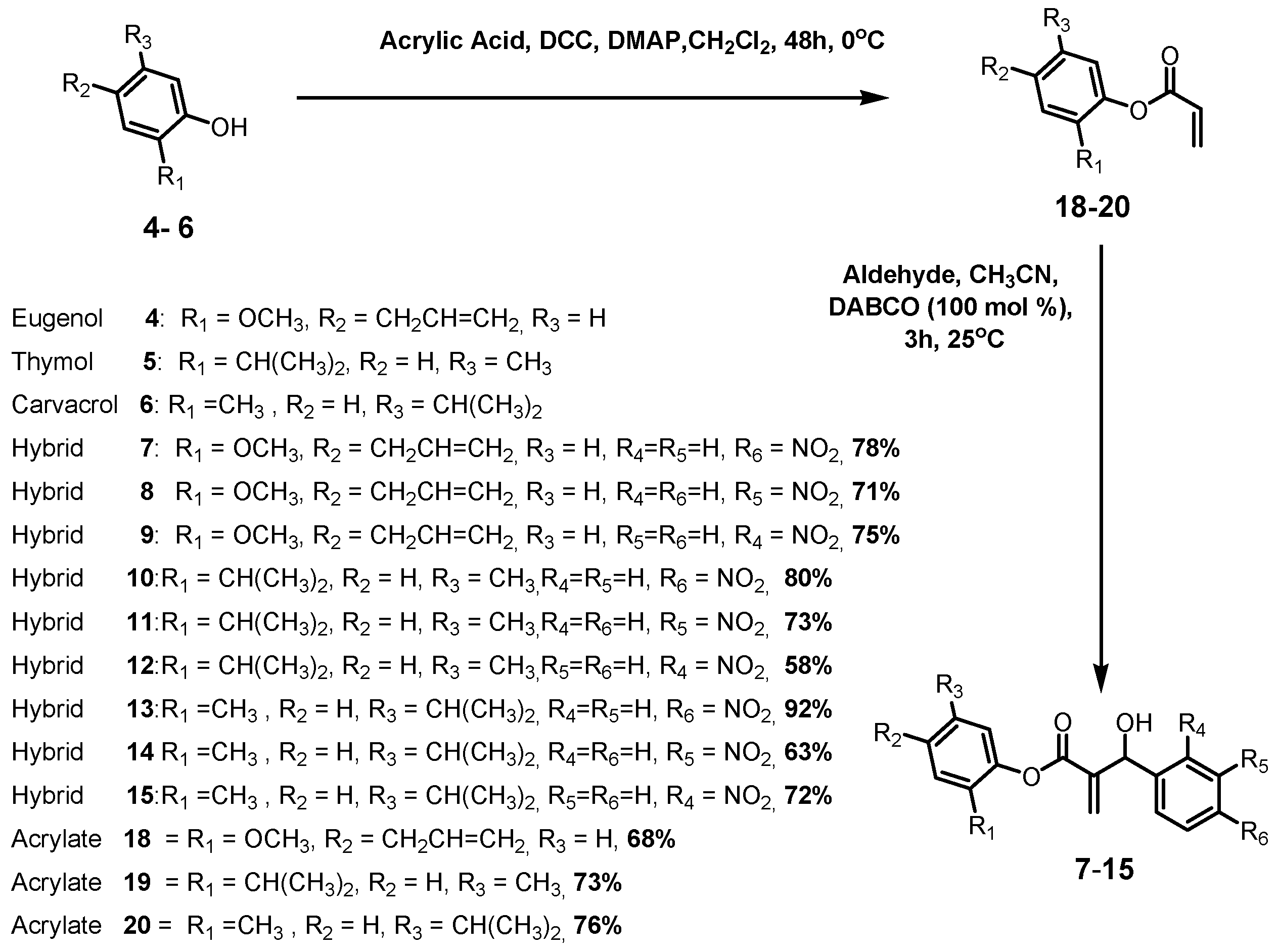
| Entry | Compounds | IC50 (μM) | HC50 (μM) | SIrb | miLogP |
|---|---|---|---|---|---|
| 1 | 4 | 77.15 ± 1.77 | >400 | >5.18 | 2.10 |
| 2 | 7 | 8.75 ± 0.44 | >400 | >45.71 | 3.05 |
| 3 | 8 | 10.49 ± 0.52 | >400 | >38.13 | 3.02 |
| 4 | 9 | 4.71 ± 0.24 | >400 | >84.92 | 3.00 |
| 5 | 5 | 115.12 ± 5.77 | - | - | 3.34 |
| 6 | 10 | 11.40 ± 0.57 | - | - | 4.05 |
| 7 | 11 | 10.56 ± 0.53 | - | - | 4.03 |
| 8 | 12 | 5.91 ± 0.30 | - | - | 4.00 |
| 9 | 6 | 138.11 ± 6.91 | - | - | 3.81 |
| 11 | 13 | 18.08 ± 0.90 | - | - | 4.52 |
| 12 | 14 | 22.30 ± 1.12 | - | - | 4.50 |
| 13 | 15 | 13.60 ± 0.68 | - | - | 4.47 |
| 14 | 16 | 15.77 ± 0.79 | >400 | >25.36 | 1.53 |
| 15 | 17 | 22.38 ± 1.12 | >400 | >17.87 | 1.50 |
| 16 | 3 | 12.06 ± 0.60 | >400 | >33.16 | 1.48 |
| 17 | Glucantime® | 1633.49 ± 437.29 | 1175.02 | 1.39 | −2.68 |
| 18 | AmphotericinB | 0.52 ± 0.03 | 11.61 | 22.34 | −2.49 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, F.J.S.; Rodrigues, K.A.d.F.; De Oliveira, R.G.; Lima Junior, C.G.; Rocha, J.D.C.; Keesen, T.S.L.; De Oliveira, M.R.; Silva, F.P.L.; Vasconcellos, M.L.A.d.A. Synthesis and In Vitro Anti Leishmania amazonensis Biological Screening of Morita-Baylis-Hillman Adducts Prepared from Eugenol, Thymol and Carvacrol. Molecules 2016, 21, 1483. https://doi.org/10.3390/molecules21111483
Xavier FJS, Rodrigues KAdF, De Oliveira RG, Lima Junior CG, Rocha JDC, Keesen TSL, De Oliveira MR, Silva FPL, Vasconcellos MLAdA. Synthesis and In Vitro Anti Leishmania amazonensis Biological Screening of Morita-Baylis-Hillman Adducts Prepared from Eugenol, Thymol and Carvacrol. Molecules. 2016; 21(11):1483. https://doi.org/10.3390/molecules21111483
Chicago/Turabian StyleXavier, Francisco José Seixas, Klinger Antonio da Franca Rodrigues, Ramon Guerra De Oliveira, Claudio Gabriel Lima Junior, Juliana Da Câmara Rocha, Tatjana Souza Lima Keesen, Marcia Rosa De Oliveira, Fábio Pedrosa Lins Silva, and Mário Luiz Araújo de Almeida Vasconcellos. 2016. "Synthesis and In Vitro Anti Leishmania amazonensis Biological Screening of Morita-Baylis-Hillman Adducts Prepared from Eugenol, Thymol and Carvacrol" Molecules 21, no. 11: 1483. https://doi.org/10.3390/molecules21111483