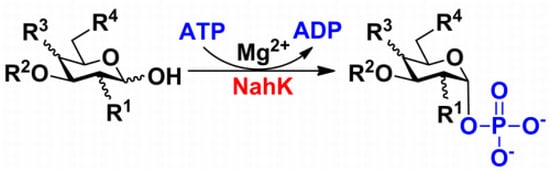
Substrate Promiscuity of N-Acetylhexosamine 1-Kinases
Abstract
:1. Introduction
2. Results and Discussion
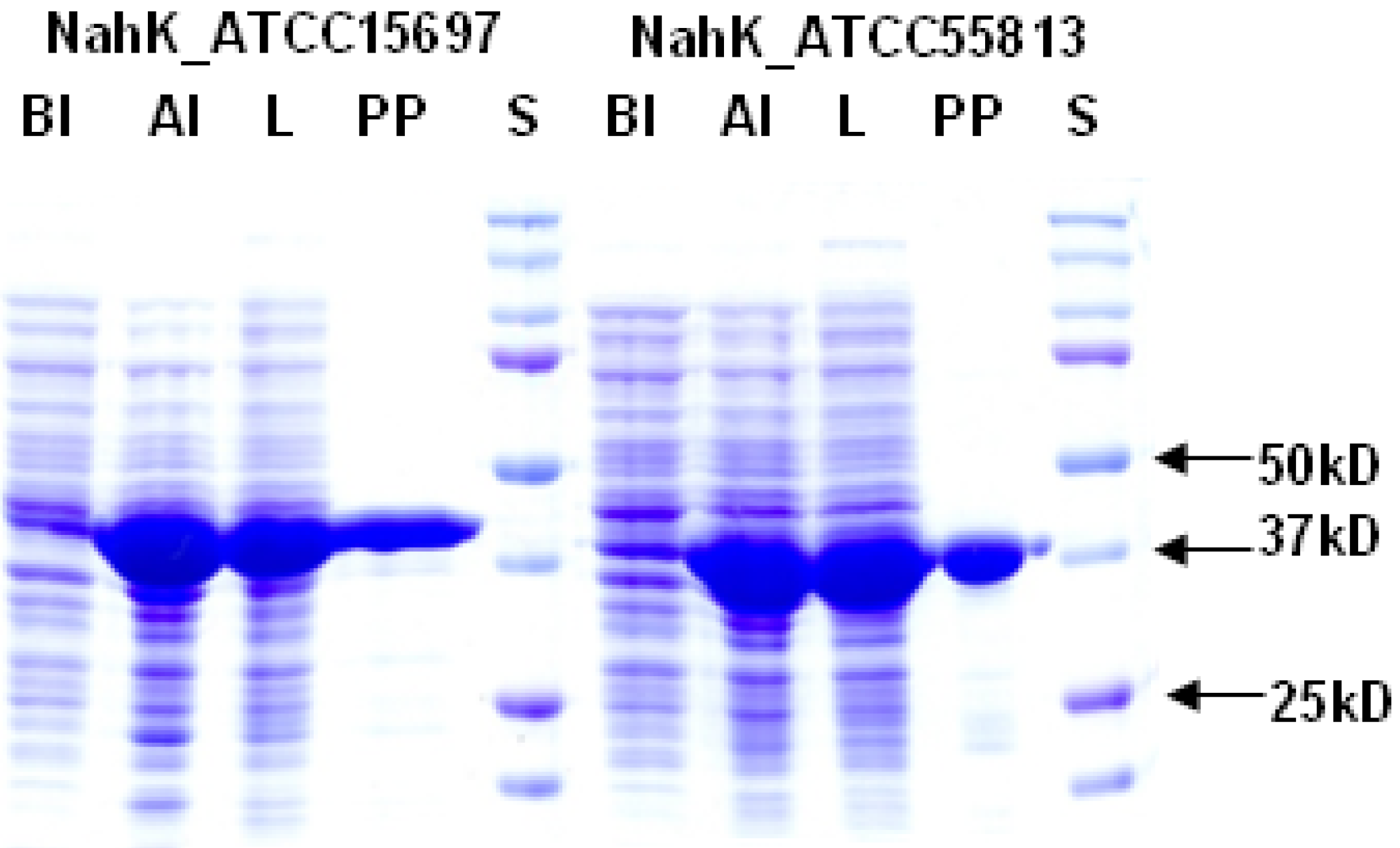
2.1. Cloning, Expression, and Purification

2.2. Capillary Electrophoresis (CE) Assays
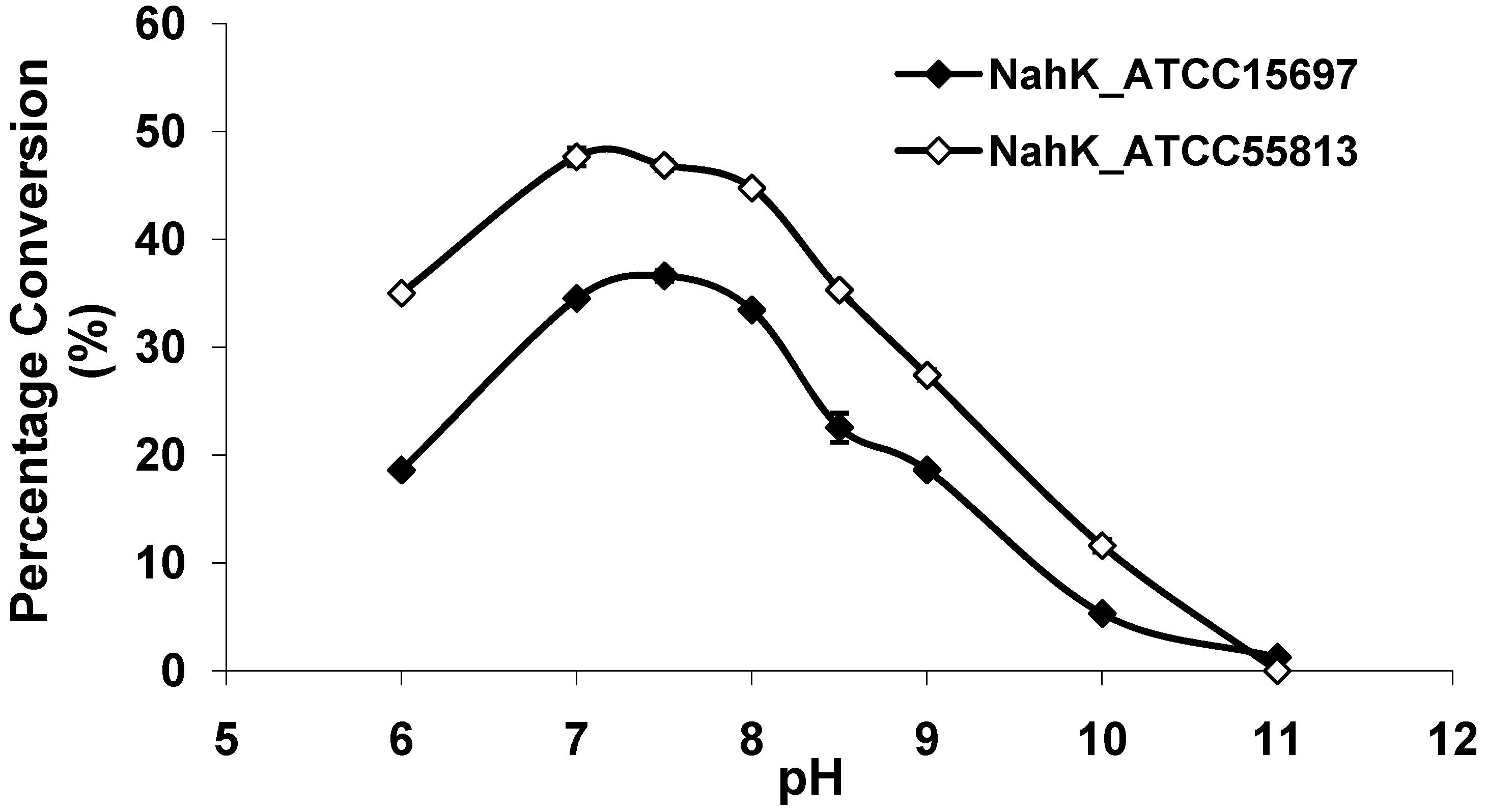
2.3. pH Profile
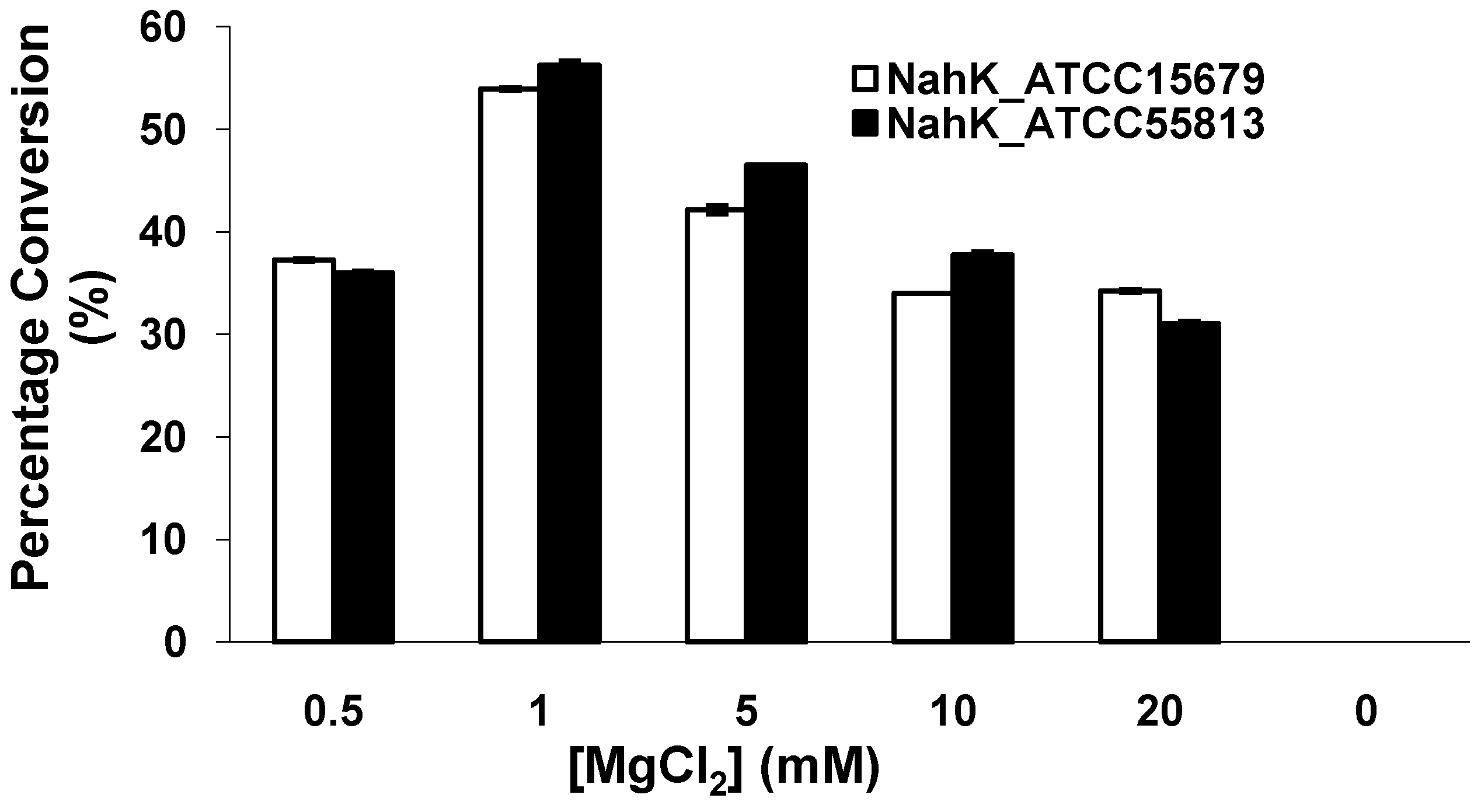
2.4. Effect of MgCl2
2.5. Kinetics
| Enzymes | NahK1_ATCC15697 | NahK_ATCC55813 | ||||
|---|---|---|---|---|---|---|
| Substrate | Km (mM) | kcat (s−1) | kcat/Km (s−1 mM−1) | Km (mM) | kcat (s−1) | kcat/Km (s−1 mM−1) |
| ATP a | 0.10 ± 0.03 | 1.1 ± 0.1 | 11.0 | 0.11 ± 0.03 | 1.3 ± 0.1 | 11.8 |
| GlcNAc | 0.06 ± 0.01 | 0.95 ± 0.01 | 15.8 | 0.06 ± 0.01 | 1.1 ± 0.1 | 18.3 |
| ATP b | 0.08 ± 0.03 | 0.38 ± 0.02 | 4.8 | 0.06 ± 0.02 | 0.48 ± 0.03 | 8.0 |
| GalNAc | 0.09 ± 0.05 | 0.46 ± 0.07 | 5.1 | 0.08 ± 0.03 | 0.57 ± 0.04 | 7.1 |
2.6. Substrate Specificity
| Substrates | Percentage Conversion (%) | Substrates | Percentage Conversion (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NahK_ATCC15697 | NahK_ATCC55813 | NahK_ATCC15697 | NahK_ATCC55813 | |||||||
| a 0.75 μM | b 15 μM | a 0.75 μM | b 15 μM | a 0.75 μM | b 15 μM | a 0.75 μM | b 15 μM | |||
 1 GlcNAc 1 GlcNAc | 35.4 ± 0.1 | NA | 42.3 ± 0.2 | NA | 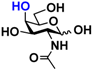 11 GalNAc 11 GalNAc | 12.5 ± 0.1 | NA | 19.9 ± 0.1 | NA | |
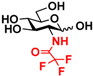 2 GlcNTFA 2 GlcNTFA | 10.7 ± 0.9 | NA | 16.2 ± 0.9 | NA | 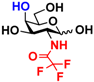 12 GalNTFA 12 GalNTFA | 11.2 ± 1.6 | NA | 21.8 ± 0.2 | NA | |
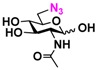
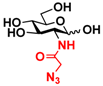 3 GlcNAcN3 3 GlcNAcN3 | 11.5 ± 1.0 | NA | 22.8 ± 0.4 | NA | 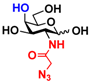 13 GalNAcN3 13 GalNAcN3 | 9.9 ± 0.6 | NA | 21.0 ± 1.2 | NA | |
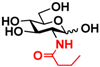 4 GlcNBu 4 GlcNBu | 20.9 ± 0.6 | NA | 35.0 ± 2.0 | NA |  14 GalNBu 14 GalNBu | 12.1 ± 0.3 | NA | 24.0 ± 0.1 | NA | |
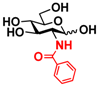 5 GlcNBz 5 GlcNBz | 10.3 ± 0.4 | NA | 5.2 ± 0.2 | NA | 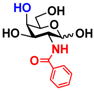 15 GalNBz 15 GalNBz | 0 | 62.2 ± 1.0 | 0 | 51.9 ± 0.5 | |
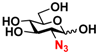 6 GlcN3 6 GlcN3 | 0 | 14.5 ± 0.1 | 0 | 7.0 ± 0.1 |  16 GalN3 16 GalN3 | 0 | 7.6 ± 0.1 | 0 | 4.3 ± 0.1 | |
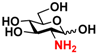 7 GlcNH2 7 GlcNH2 | 0 | 15.0 ± 0.1 | 0 | 8.4 ± 0.1 | 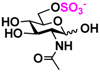 17 GlcNAc6S 17 GlcNAc6S | 0 | 11.7 ± 0.2 | 0 | 6.6 ± 0.1 | |
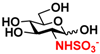 8 GlcNS 8 GlcNS | 0 | 6.4 ± 0.2 | 0 | 4.0 ± 0.1 | 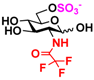 18 GlcNTFA6S 18 GlcNTFA6S | 0 | 7.2 ± 0.1 | 0 | 3.3 ± 0.2 | |
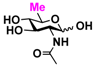 9 GlcNAc6Me 9 GlcNAc6Me | 4.4 ± 1.2 | 41.8 ± 0.3 | 2.1 ± 0.2 | 36.3 ± 0.3 | 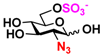 19 GlcN36S 19 GlcN36S | 0 | 6.9 ± 0.1 | 0 | 4.4 ± 0.1 | |
 10 GlcNAc6N3 10 GlcNAc6N3 | 0 | 37.2 ± 0.5 | 0 | 23.4 ± 0.1 | 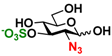 20 GlcN33S 20 GlcN33S | 0 | 4.9 ± 0.1 | 0 | 3.9 ± 0.1 | |
| Substrates | Percentage Conversion (%) | Substrates | Percentage Conversion (%) | ||
|---|---|---|---|---|---|
| NahK_ATCC15697 | NahK_ATCC55813 | NahK_ATCC15697 | NahK_ATCC55813 | ||
 21 Glc 21 Glc | 9.1 ± 0.1 | 4.7 ± 0.1 | 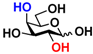 28 Gal 28 Gal | 7.3 ± 0.2 | 4.4 ± 0.1 |
 22 2-deoxyGlc 22 2-deoxyGlc | 44.8 ± 0.2 | 28.4 ± 0.1 |  29 ManNAc 29 ManNAc | 8.9 ± 0.1 | 5.5 ± 0.1 |
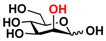 23 Man 23 Man | 68.0 ± 1.7 | 37.1 ± 0.4 | 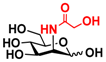 30 ManNGc 30 ManNGc | 7.6 ± 0.1 | 5.4 ± 0.2 |
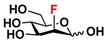 24 2F-Man 24 2F-Man | 44.4 ± 0.2 | 47.0 ± 0.1 | 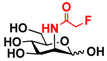 31 ManNAcF 31 ManNAcF | 12.0 ± 0.1 | 9.1 ± 0.2 |
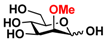 25 2Me-Man 25 2Me-Man | 9.4 ± 0.5 | 0 | 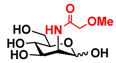 32 ManNAcOMe 32 ManNAcOMe | 12.0 ± 0.4 | 7.4 ± 0.3 |
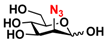 26 2N3-Man 26 2N3-Man | 53.3 ± 0.1 | 40.2 ± 0.2 | 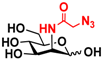 33 ManNAcN3 33 ManNAcN3 | 20.3 ± 0.3 | 18.6 ± 0.4 |
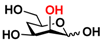 27 4-deoxyMan 27 4-deoxyMan | 37.1 ± 0.2 | 23.9 ± 0.1 |  34 ManNAc6OMe 34 ManNAc6OMe | 32.6 ± 0.1 | 28.9 ± 0.1 |
3. Experimental
3.1. Bacterial Strains, Plasmids, and Materials
3.2. Cloning
3.3. Expression and Purification
3.4. Quantification of Purified Protein
3.5. pH Profile by Capillary Electrophoresis (CE) Assays
3.6. Effect of MgCl2 on the Enzymatic Activity
3.7. Substrates Specificity Assays
3.8. Kinetics by CE Assays
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Bakkers, J.; Semino, C.E.; Stroband, H.; Kijne, J.W.; Robbins, P.W.; Spaink, H.P. An important developmental role for oligosaccharides during early embryogenesis of cyprinid fish. Proc. Natl. Acad. Sci. USA 1997, 94, 7982–7986. [Google Scholar] [CrossRef]
- Gooday, G.W. The ecology of chitin degradation. Adv. Microb. Ecol. 1990, 11, 387–430. [Google Scholar] [CrossRef]
- Siddiqui, I.R.; Wood, P.J. Structural investigation of oxalate-soluble rapeseed (Brassica campestris) polysaccharides. 3. An arabinan. Carbohydr. Res. 1974, 36, 35–44. [Google Scholar] [CrossRef]
- Barreteau, H.; Kovac, A.; Boniface, A.; Sova, M.; Gobec, S.; Blanot, D. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 168–207. [Google Scholar] [CrossRef]
- Yu, H.; Chen, X. Carbohydrate post-glycosylational modifications. Org. Biomol. Chem. 2007, 5, 865–872. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar]
- Salmivirta, M.; Lidholt, K.; Lindahl, U. Heparan sulfate: A piece of information. FASEB J. 1996, 10, 1270–1279. [Google Scholar]
- Hart, G.W.; Housley, M.P.; Slawson, C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007, 446, 1017–1022. [Google Scholar] [CrossRef]
- Buscaglia, C.A.; Campo, V.A.; Frasch, A.C.; Di Noia, J.M. Trypanosoma cruzi surface mucins: Host-dependent coat diversity. Nat. Rev. Microbiol. 2006, 4, 229–236. [Google Scholar]
- Nishimoto, M.; Kitaoka, M. Identification of N-acetylhexosamine 1-kinase in the complete lacto-N-biose I/galacto-N-biose metabolic pathway in Bifidobacterium longum. Appl. Environ. Microbiol. 2007, 73, 6444–6449. [Google Scholar] [CrossRef]
- Cai, L.; Guan, W.; Kitaoka, M.; Shen, J.; Xia, C.; Chen, W.; Wang, P.G. A chemoenzymatic route to N-acetylglucosamine-1-phosphate analogues: Substrate specificity investigations of N-acetylhexosamine 1-kinase. Chem. Commun. (Camb) 2009, 2944–2946. [Google Scholar]
- Cai, L.; Guan, W.; Wang, W.; Zhao, W.; Kitaoka, M.; Shen, J.; O'Neil, C.; Wang, P.G. Substrate specificity of N-acetylhexosamine kinase towards N-acetylgalactosamine derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 5433–5435. [Google Scholar] [CrossRef]
- Fang, J.; Guan, W.; Cai, L.; Gu, G.; Liu, X.; Wang, P.G. Systematic study on the broad nucleotide triphosphate specificity of the pyrophosphorylase domain of the N-acetylglucosamine-1-phosphate uridyltransferase from Escherichia coli K12. Bioorg. Med. Chem. Lett. 2009, 19, 6429–6432. [Google Scholar] [CrossRef]
- Zhao, G.; Guan, W.; Cai, L.; Wang, P.G. Enzymatic route to preparative-scale synthesis of UDP-GlcNAc/GalNAc, their analogues and GDP-fucose. Nat. Protoc. 2010, 5, 636–646. [Google Scholar] [CrossRef]
- Guan, W.; Cai, L.; Wang, P.G. Highly efficient synthesis of UDP-GalNAc/GlcNAc analogues with promiscuous recombinant human UDP-GalNAc pyrophosphorylase AGX1. Chemistry (Weinheim an der Bergstrasse, Germany) 2010, 16, 13343–13345. [Google Scholar] [CrossRef]
- Lau, K.; Thon, V.; Yu, H.; Ding, L.; Chen, Y.; Muthana, M.M.; Wong, D.; Huang, R.; Chen, X. Highly efficient chemoenzymatic synthesis of beta1-4-linked galactosides with promiscuous bacterial beta1-4-galactosyltransferases. Chem. Commun. (Camb) 2010, 46, 6066–6068. [Google Scholar]
- Yu, H.; Thon, V.; Lau, K.; Cai, L.; Chen, Y.; Mu, S.; Li, Y.; Wang, P.G.; Chen, X. Highly efficient chemoenzymatic synthesis of beta1-3-linked galactosides. Chem. Commun. (Camb) 2010, 46, 7507–7509. [Google Scholar] [CrossRef]
- Cao, H.; Li, Y.; Lau, K.; Muthana, S.; Yu, H.; Cheng, J.; Chokhawala, H.A.; Sugiarto, G.; Zhang, L.; Chen, X. Sialidase substrate specificity studies using chemoenzymatically synthesized sialosides containing C5-modified sialic acids. Org. Biomol. Chem. 2009, 7, 5137–5145. [Google Scholar] [CrossRef]
- Nishio, T.; Miyake, Y.; Kubota, K.; Yamai, M.; Miki, S.; Ito, T.; Oku, T. Synthesis of the 4-, 6-deoxy, and 4,6-dideoxy derivatives of D-mannose. Carbohydr. Res. 1996, 280, 357–363. [Google Scholar] [CrossRef]
- Song, X.; Yu, H.; Chen, X.; Lasanajak, Y.; Tappert, M.M.; Air, G.M.; Tiwari, V.K.; Cao, H.; Chokhawala, H.A.; Zheng, H.; et al. A sialylated glycan microarray reveals novel interactions of modified sialic acids with proteins and viruses. J. Biol. Chem. 2011. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 1–34 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.; Yu, H.; Chen, Y.; Lau, K.; Cai, L.; Cao, H.; Tiwari, V.K.; Qu, J.; Thon, V.; Wang, P.G.; et al. Substrate Promiscuity of N-Acetylhexosamine 1-Kinases. Molecules 2011, 16, 6396-6407. https://doi.org/10.3390/molecules16086396
Li Y, Yu H, Chen Y, Lau K, Cai L, Cao H, Tiwari VK, Qu J, Thon V, Wang PG, et al. Substrate Promiscuity of N-Acetylhexosamine 1-Kinases. Molecules. 2011; 16(8):6396-6407. https://doi.org/10.3390/molecules16086396
Chicago/Turabian StyleLi, Yanhong, Hai Yu, Yi Chen, Kam Lau, Li Cai, Hongzhi Cao, Vinod Kumar Tiwari, Jingyao Qu, Vireak Thon, Peng George Wang, and et al. 2011. "Substrate Promiscuity of N-Acetylhexosamine 1-Kinases" Molecules 16, no. 8: 6396-6407. https://doi.org/10.3390/molecules16086396