Synthesis and Toxicity Evaluation of Some N4-Aryl Substituted 5-Trifluoromethoxyisatin-3-thiosemicarbazones
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
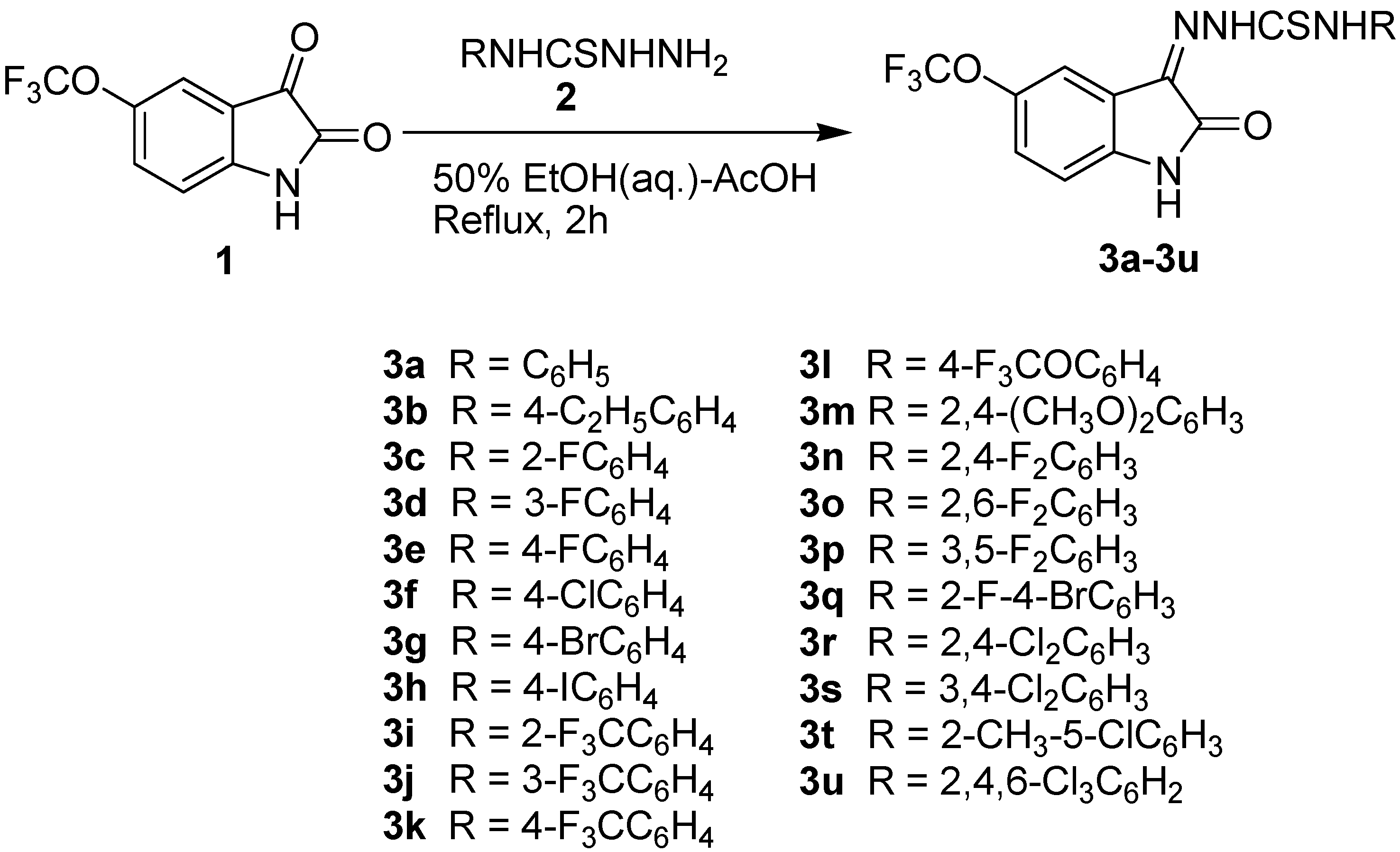
2.2. Toxicity
| Compounds | LD50 (M) |
|---|---|
| 3a | 4.49 × 10−5 |
| 3b | >2.45 × 10−4 |
| 3c | >2.51 × 10−4 |
| 3d | >2.51 × 10−4 |
| 3e | 3.72 × 10−5 |
| 3f | >2.42 × 10−4 |
| 3g | >2.18 × 10−4 |
| 3h | >1.98 × 10−4 |
| 3i | 1.80 × 10−4 |
| 3j | 1.70 × 10−4 |
| 3k | 1.11 × 10−5 |
| 3l | 1.81 × 10−5 |
| 3m | >2.27 × 10−4 |
| 3n | 1.43 × 10−5 |
| 3o | 1.34 × 10−5 |
| 3p | 4.32 × 10−5 |
| 3q | 1.60 × 10−4 |
| 3r | >2.23 × 10−4 |
| 3s | >2.23 × 10−4 |
| 3t | >2.34 × 10−4 |
| 3u | >2.07 × 10−4 |
| 2-(2-Oxo-1,2-dihydro-3H-indol-3-ylidene)-N-phenyl-1-hydrazinecarbothioamide * | >3.38 × 10−4 |
3. Experimental
3.1. General
3.2. Synthesis
3.2.1. General Procedure for the Preparation of 5-Ttrifluoromethoxyisatin-thiosemicarbazones 3a-3u
3.3. Bioassay of Toxic Activity
4. Conclusions
Acknowledgments
Conflict of Interest
Supplementary Materials
Supplementary File 1References and Notes
- da Silva, J.F.M.; Garden, S.J.; Pinto, A. da C. The chemistry of isatins: A review from 1975 to 1999. J. Braz. Chem. Soc. 2001, 12, 273–324, and references therein. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Smitha, S.; Jyoti, M.; Sridhar, S.K. Biological activities of isatin and its derivatives. Acta Pharm. 2005, 55, 27–46, and references therein. [Google Scholar]
- Vine, K.L.; Matesic, L.; Locke, J.M.; Ranson, M.; Skropeta, D. Cytotoxic and anticancer activities of isatin and its derivatives: A comprehensive review from 2000-2008. Anti-Cancer Agents Med. Chem. 2009, 9, 397–414, and references therein. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Bin-Jubair, F.A.S. Anti-tubercular activity of isatin derivatives. Int. J. Res. Pharm. Sci. 2010, 1, 113–126, and references therein. [Google Scholar]
- Chiyanzu, I.; Hansell, E.; Gut, J.; Rosenthal, P.J.; McKerrow, J.H.; Chibale, K. Synthesis and evaluation of isatins and thiosemicarbazone derivatives against cruzain, falcipain-2 and rhodesain. Bioorg. Med. Chem. Lett. 2003, 13, 3527–3530. [Google Scholar] [CrossRef]
- Chiyanzu, I.; Clarkson, C.; Smith, P.J.; Lehman, J.; Gut, J.; Rosenthal, P.J.; Chibale, K. Design, synthesis and anti-plasmodial evaluation in vitro of new 4-aminoquinoline isatin derivatives. Bioorg. Med. Chem. 2005, 13, 3249–3261. [Google Scholar] [CrossRef]
- Chen, L.R.; Wang, Y.C.; Lin, Y.W.; Chou, S.Y.; Chen, S.F.; Liu, L.T.; Wu, Y.T.; Kuo, C.J.; Chen, T.S.; Juang, S.H. Synthesis and evaluation of isatin derivatives as effective SARS coronavirus 3CL protease inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 3058–3062. [Google Scholar] [CrossRef]
- Bal, T.R.; Anand, B.; Yogeeswari, P.; Sriram, D. Synthesis and evaluation of anti-HIV activity of isatin β-thiosemicarbazone derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 4451–4455. [Google Scholar]
- Pirrung, M.C.; Pansare, S.V.; Sarma, K.D.; Keith, K.A.; Kern, E.R. Combinatorial optimization of isatin-β-thiosemicarbazones as anti-poxvirus agents. J. Med. Chem. 2005, 48, 3045–3050. [Google Scholar] [CrossRef]
- Patel, A.; Bari, S.; Talele, G.; Patel, J.; Sarangapani, M. Synthesis and antimicrobial activity of some new isatin derivatives. Iran. J. Pharm. Res. 2006, 4, 249–254. [Google Scholar]
- Beauchard, A.; Ferandin, Y.; Frere, S.; Lozach, O.; Blairvacq, M.; Meijer, L.; Thiery, V.; Besson, T. Synthesis of novel 5-substituted indirubins as protein kinases inhibitors. Bioorg. Med. Chem. 2006, 14, 6434–6443. [Google Scholar]
- Terzioglu, N.; Karali, N.; Gursoy, A.; Pannecouque, C.; Leysen, P.; Paeshuyse, J.; Neyts, J.; de Clercq, E. Synthesis and primary antiviral activity evaluation of 3-hydrazono-5-nitro-2-indolinone derivatives. ARKIVOC 2006, i, 109–118. [Google Scholar]
- Hyatt, J.L.; Moak, T.; Hatfield, M.J.; Tsurkan, L.; Edwards, C.C.; Wierdl, M.; Danks, M.K.; Wadkins, R.M.; Potter, P.M. Selective inhibition of carboxylesterases by isatins, indole-2,3-diones. J. Med. Chem. 2007, 50, 1876–1885. [Google Scholar] [CrossRef]
- Ravichandran, V.; Mohan, S.; Kumar, K.S. Synthesis and antimicrobial activity of Mannich bases of isatin and its derivatives with 2-[(2,6-dichlorophenyl)amino]phenylacetic acid. ARKIVOC 2007, xiv, 51–57. [Google Scholar]
- Guzel, O.; Karali, N.; Salman, A. Synthesis and antituberculosis activity of 5-methyl/trifluoromethoxy-1H-indole-2,3-dione 3-thiosemicarbazone derivatives. Bioorg. Med. Chem. 2008, 16, 8976–8987. [Google Scholar] [CrossRef]
- Smitha, S.; Pandeya, S.N.; Stables, J.P.; Ganapathy, S. Anticonvulsant and sedative-hypnotic activeties of N-acetyl/methyl isatin derivatives. Sci. Pharm. 2008, 76, 621–636. [Google Scholar] [CrossRef]
- Singh, U.K.; Pandeya, S.N.; Singh, A.; Srivastava, B.K.; Pandey, M. Synthesis and antimicrobial activity of Schiff’s and N-Mannich bases of isatin and its derivatives with 4-amino-N-carbamimidoyl benzene sulfonamide. Int. J. Pharm. Sci. Drug Res. 2010, 2, 151–154. [Google Scholar]
- Banerjee, D.; Yogeeswari, P.; Bhat, P.; Thomas, A.; Srividya, M.; Sriram, D. Novel isatinyl thiosemicarbazones derivatives as potential molecule to combat HIV-TB co-infection. Eur. J. Med. Chem. 2011, 46, 106–121. [Google Scholar] [CrossRef]
- Quenelle, D.C.; Keith, K.A.; Kern, E.R. In vitro and in vivo evaluation of isatin-β-thiosemicarbazone and marboran against vaccinia and cowpox virus infections. Antivir. Res. 2006, 71, 24–30. [Google Scholar]
- Hall, M.D.; Salam, N.K.; Hellawell, J.L.; Fales, H.M.; Kensler, C.B.; Ludwig, J.A.; Szakacs, G.; Hibbs, D.E.; Gottesman, M.M. Synthesis, activity, and pharmacophore development for isatin-β-thiosemicarbazones with selective activity toward multidrug-resistant cells. J. Med. Chem. 2009, 52, 3191–3204. [Google Scholar] [CrossRef]
- Pervez, H.; Iqbal, M.S.; Tahir, M.Y.; Choudhary, M.I.; Khan, K.M. Synthesis of some N4-substituted isatin-3-thiosemicarbazones. Nat. Prod. Res. 2007, 21, 1178–1186. [Google Scholar] [CrossRef]
- Pervez, H.; Iqbal, M.S.; Tahir, M.Y.; Nasim, F.H.; Choudhary, M.I.; Khan, K.M. In vitro cytotoxic, antibacterial, antifungal and urease inhibitory activities of some N4-substituted isatin-3-thiosemicarbazones. J. Enzyme Inhib. Med. Chem. 2008, 23, 848–854. [Google Scholar] [CrossRef]
- Pervez, H.; Chohan, Z.H.; Ramzan, M.; Nasim, F.H.; Khan, K.M. Synthesis and biological evaluation of some new N4-substituted isatin-3-thiosemicarbazones. J. Enzyme Inhib. Med. Chem. 2009, 24, 437–446. [Google Scholar]
- Pervez, H.; Manzoor, N.; Yaqub, M.; Khan, A.; Khan, K.M.; Nasim, F.H.; Choudhary, M.I. Synthesis and urease inhibitory properties of some new N4-substituted 5-nitroisatin-3-thiosemicarbazones. Lett. Drug. Des. Discov. 2010, 7, 102–108. [Google Scholar]
- Pervez, H.; Ramzan, M.; Yaqub, M.; Khan, K.M. Synthesis, cytotoxic and phytotoxic effects of some new N4-aryl substituted isatin-3-thiosemicarbazones. Lett. Drug. Des. Discov. 2011, 8, 452–458. [Google Scholar]
- Karali, N. Synthesis and primary cytotoxicity evaluation of new 5-nitroindole-2,3-dione derivatives. Eur. J. Med. Chem. 2002, 37, 909–918. [Google Scholar] [CrossRef]
- Karali, N.; Terzioglu, N.; Gursoy, A. Synthesis and primary cytotoxicity evaluation of new 5-bromo-3-substituted hydrazono-1H-2-indolinones. Arch. Pharm. 2002, 335, 374–380. [Google Scholar] [CrossRef]
- Omar, A.-M.E.; Eshba, N.H.; Salama, H.M. Synthesis of some substituted isatin-β-thio-semicarbazones and isatin-β-hydrazonothiazoline derivatives as potential antiviral and antimicrobial agents. Arch. Pharm. 1984, 317, 701–709. [Google Scholar]
- Petrov, I.; Grupce, O.; Stafilov, T. The nitrogen-hydrogen stretching region of some imides and thioimides. J. Mol. Struct. 1986, 142, 275–278. [Google Scholar] [CrossRef]
- Naumov, P.; Anastasova, F. Experimental and theoretical vibrational study of isatin, its 5-(NO2, F, Cl, Br, I, CH3) analogues and the isatinato anion. Spectrochim. Acta 2001, 57A, 469–481. [Google Scholar]
- Nizamuddin, N.; Khan, M.H.; Alauddin, S.; Haque, R. Synthesis and fungicidal activity of some 2-arylamino-1,3,4-thiadiazino[6,5-b]indoles and 2-aryl-1,3,4-oxadiazolo-[2,3-c]-1,2,4-triazino[5,6-b]indoles. Indian J. Chem. 1999, 38B, 501–504. [Google Scholar]
- Laatsch, H.; Thomson, R.H.; Cox, P.J. Spectroscopic properties of violacein and related compounds: Crystal structure of tetramethyl-violacein. J. Chem. Soc. Perkin Trans. 2 1984, 1331–1339. [Google Scholar]
- Pervez, H.; Iqbal, M.S.; Saira, N.; Yaqub, M.; Tahir, M.N. 4-(5-Chloro-2-methylphenyl)-1-[2-oxo-5-(trifluoromethoxy)indolin-3-ylidene]thiosemicarbazide. Acta Cryst. 2010, E66, o1169–o1170. [Google Scholar]
- Pervez, H.; Iqbal, M.S.; Saira, N.; Yaqub, M.; Tahir, M.N. 1-[2-Oxo-5-(trifluoromethoxy)indolin-3-ylidene]-4-[4-(trifluoromethyl)phenyl]thiosemicarbazide. Acta Cryst. 2010, E66, o1749. [Google Scholar]
- McLaughlin, J.L.; Chang, C.J.; Smith, D.L. Bench Top Bioassays for the Discovery of Bioactive Natural Products: An Update. In Studies in Natural Products Chemistry: Structure and Chemistry (Part B); Rahman, A.U., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 9, pp. 383–409. [Google Scholar]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nicholas, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed; Cambridge University Press: London, UK, 1971. [Google Scholar]
- Sample Availability: Samples of the compounds 3a-3u are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pervez, H.; Saira, N.; Iqbal, M.S.; Yaqub, M.; Khan, K.M. Synthesis and Toxicity Evaluation of Some N4-Aryl Substituted 5-Trifluoromethoxyisatin-3-thiosemicarbazones. Molecules 2011, 16, 6408-6421. https://doi.org/10.3390/molecules16086408
Pervez H, Saira N, Iqbal MS, Yaqub M, Khan KM. Synthesis and Toxicity Evaluation of Some N4-Aryl Substituted 5-Trifluoromethoxyisatin-3-thiosemicarbazones. Molecules. 2011; 16(8):6408-6421. https://doi.org/10.3390/molecules16086408
Chicago/Turabian StylePervez, Humayun, Naveeda Saira, Mohammad Saeed Iqbal, Muhammad Yaqub, and Khalid Mohammed Khan. 2011. "Synthesis and Toxicity Evaluation of Some N4-Aryl Substituted 5-Trifluoromethoxyisatin-3-thiosemicarbazones" Molecules 16, no. 8: 6408-6421. https://doi.org/10.3390/molecules16086408
APA StylePervez, H., Saira, N., Iqbal, M. S., Yaqub, M., & Khan, K. M. (2011). Synthesis and Toxicity Evaluation of Some N4-Aryl Substituted 5-Trifluoromethoxyisatin-3-thiosemicarbazones. Molecules, 16(8), 6408-6421. https://doi.org/10.3390/molecules16086408




