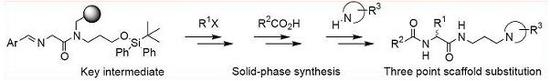
General procedures for manual solid-phase organic syntheses
Preparation of MBHA-BAL Resin 4: PL-MBHA·HCl resin (1.03 g, 1.65 mmol, 1.60 mmol/g) loaded in a 50 mL peptide synthesis reaction vessel was sequentially washed with DMF (3 × 17 mL), CH
2Cl
2 (2 × 17 mL), 10% DIEA/CH
2Cl
2 (5 × 17 mL) and CH
2Cl
2 (2 × 17 mL). A solution of 4-(4-formyl-3,5-dimethoxyphenoxy)butyric acid (1.77 g, 6.60 mmol, 4 equiv), HBTU (2.50 g, 6.60 mmol, 4 equiv) and DIEA (2.30 mL, 13.2 mmol, 8 equiv) in anhydrous DMF (23 mL) prepared immediately prior to the reaction was then added to the washed resin. The reaction mixture was agitated on an orbital shaker for 18 h. The completion of the reaction was confirmed by a negative chloranil test [
15]. The resultant orange yellow MBHA-BAL resin
4 was washed with DMF (3 × 17 mL) and CH
2Cl
2 (4 × 17 mL), and dried under low vacuum for 24 h.
Preparation of the Secondary Amine-Bound Resin 21 by Reductive Amination: The dried MBHA-BAL resin
4 (1.51 g, 1.65 mmol) was swelled in CH
2Cl
2 (17 mL) for 1 h, followed by DMF washes (3 × 17 mL).
O-
tert-Butyldiphenylsilyl-3-aminopropanol hydrochloride [
13] (8.66 g, 24.75 mmol, 15 equiv) was added as a solid followed by addition of DMF–TMOF (1:2, 28 mL) to the resin. Theresulting reaction mixture was heated to 60 °C for 24 h. The resin-bound Schiff base product was washed with DMF (4 × 17 mL), THF (5 × 17 mL), MeOH (2 × 17 mL), CH
2Cl
2 (2 × 17 mL), and MeOH (3 × 17 mL). After the resulting imine resin was swelled in CH
2Cl
2 (17 mL) for 30 min followed by THF (17 mL) wash, it was treated with a solution of NaBH
3CN (1.04 g, 16.5 mmol, 10 equiv) in 5% HOAc in THF–MeOH (1:1, 28 mL). The reaction mixture was agitated on an orbital shaker for 18 h. The resin was drained and washed with THF–MeOH (1:1, 3 × 17 mL), MeOH (2 × 17 mL), THF (3 × 17 mL) and CH
2Cl
2 (3 × 17 mL). The amine resin product
21 was air-dried and was then used directly for subsequent coupling reactions.
Coupling of the Amine Resin 21 with N-Fmoc-Glycine using HBTU to Prepare Resin-Bound 22: The amine resin
21 (1.65 mmol) was swelled in CH
2Cl
2 (17 mL) for 30 min, and then it was washed with CH
2Cl
2–DMF (85:15, 17 mL). Fmoc-Gly-OH (2.45 g, 8.25 mmol, 5 equiv), HBTU (3.13 g, 8.25 mmol, 5 equiv), CH
2Cl
2–DMF (85:15, 39 mL) and DIEA (2.87 mL, 16.5 mmol, 10 equiv) were then added to the reaction vessel. The reaction mixture was agitated on an orbital shaker for 42 h. The completion of the reaction was indicated by a negative chloranil test [
15]. The resulting Fmoc-Gly-BAL resin product
22 was then washed with DMF (3 × 17 mL), THF–MeOH (1:1, 3 × 17 mL), THF (3 × 17 mL), and CH
2Cl
2 (3 × 17 mL). The washed resin
22 was dried under low vacuum for 24–36 h.
Preparation of the 3,4-Dichlorobenzaldehyde Imine of Glycine on BAL Resin 23: The resin 22 (1.65 mmol) was swelled in CH2Cl2 (17 mL) for 30 min, and washed with DMF (5 × 17 mL), then it was treated with 20% piperidine/DMF (23 mL, 20 min) and subsequently washed with DMF (6 × 17 mL). The resin was swelled in CH2Cl2 (17 mL) for 30 min and washed with NMP (4 × 17 mL). A solution of 3,4-dichlorobenzaldehyde (1.25 M, 19.8 mL, 24.75 mmol, 15 equiv) in NMP–TMOF (1:2) was then added to the reaction vessel and the reaction mixture was rotated for 24 h. The resulting resin-bound Schiff base product 23 was sequentially washed with NMP (4 × 17 mL), THF (3 × 17 mL), CH2Cl2 (3 × 17 mL) and dried under low vacuum for 24 h.
Alkylation of the Aldimine of Glycine on BAL Resin 23 with Alkyl Halides, Subsequent Imine Hydrolysis, and Acylation with Carboxylic Acids: After resin-bound Schiff base
23 (50 μmol) was pre-swelled in CH
2Cl
2 for 1 h, it was washed with NMP (4 × 1 mL). To the resin was then added a solution of R
1X in NMP (2.0 M, 0.25 mL, 0.5 mmol, 10 equiv), followed by addition of BTPP solution in NMP (2.0 M, 0.25 mL, 0.5 mmol, 10 equiv). Alkylation was allowed to proceed at ambient temperature for 24 h with rotation. The alkylated resin product was filtered and washed with NMP (4 × 1 mL), CH
2Cl
2 (4 × 1 mL) and THF (3 × 1 mL). The resin was then treated with 1N HCl–THF (1:2, 1 mL) for 20 min. The resulting resin was filtered and washed with THF (3 × 1 mL), 10% DIEA/CH
2Cl
2 (5 × 1 mL), CH
2Cl
2 (2 × 1 mL). After the resin was swelled in CH
2Cl
2 (1 mL) for 1 h, and washed with DMF (3 × 1 mL), to the resin was added a solution of carboxylic acid R
2COOH and HOBt in DMF (0.38 M, 0.66 mL, 0.25 mmol, 5 equiv), which was pre-mixed (6 - 10 min before addition) with DIPCDI (neat, 32 mg, 0.25 mmol, 5 equiv). The reaction mixture was allowed to rotate for 18 h. The completion of the reaction was confirmed by a negative chloranil test [
15], and the filtered resin product
25 was washed with DMF (4 × 1 mL), THF (3 × 1 mL), CH
2Cl
2 (3 × 1 mL), and dried in air.
Model Studies for the Synthesis of 2a–2d via Deprotection of Silyl Ether 25 with TBAF, Subsequent Mesylation of Alcohol 26 using Mesyl Chloride in Pyridine, and N-Alkylation with Tetrahydroisoquinoline: The acylated resin product 25 (50 μmol) was washed with THF (3 × 1 mL), and swelled in THF for 1 h. TBAF (1 M in THF, 1.0 mL, 1.0 mmol, 20 equiv) was added to the drained resin, and the reaction mixture was allowed to rotate for 18 h. The drained resin was then washed with THF (5 × 1 mL) and CH2Cl2 (3 × 1 mL). After the alcohol resin 26 was swelled in CH2Cl2 for 1 h, a suspension of anhydrous pyridine with MsCl (1 M, 1.0 mL, 0.5 mmol, 20 equiv) was added to the resin, and the resulting mixture was rotated for 1 h. The drained resin product 27a was washed with DMF (3 × 1 mL), H2O (1 × 1 mL), DMF (2 × 1 mL) and CH2Cl2 (5 × 1 mL). After the air-dried resin was swelled in CH2Cl2 for 30 min, to the resin was added a solution of tetrahydroisoquinoline in DMSO (1 M, 0.75 mL, 0.75 mmol, 15 equiv), and the reaction mixture was heated to 80 °C for 5 h. The resulting resin product was drained, washed with DMF (2 × 1 mL), H2O (2 × 1 mL), DMF (3 × 1 mL), CH2Cl2 (4 × 1 mL), and air-dried. The resin product was then cleaved with 50% TFA/CH2Cl2 (1 mL) over 1.5 h, and the filtrate of the reaction mixture was collected and combined with washes of CH2Cl2 (2 × 1 mL) of the resin. A 100 μL sample of the combined solution was analyzed for crude purity by LC/MS. The cleavage solution was evaporated with a stream of nitrogen in a contained system with trapping of the evaporated TFA in 2N NaOH. The crude residue was re-dissolved in CH2Cl2, or CH2Cl2 with MeOH, if needed (total solution volume ≤0.5 mL), and purified using a pre-loaded silica gel cartridge with CH2Cl2–MeOH (95:5 or 93:7) to elute the purified product. Following solvent removal under N2 flow, the purified product 2a–2d was normally obtained as an amorphous white solid or light yellow oil.
α-[(4-Cyanobenzoyl)amino]-N-[(3,4-dihydroisoquinolin-2(1H)-yl)propyl]-benzenepropanamide (2a): Product 2a was obtained from resin 23, benzyl bromide, 4-cyanobenzoic acid, and tetrahydroisoquinoline as an amorphous white solid (6 mg, 26% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 65%, tR = 7.3 min; 1H-NMR (CD3OD/CDCl3): δ 1.67–1.78 (m, 2H), 2.49–2.61 (m, 3H), 2.74–2.86 (m, 2H), 2.88–2.93 (m, 1H), 2.96 (dd, J = 8.0 Hz, J = 13.5 Hz, 1H), 3.03 (dd, J = 6.0 Hz, J = 13.5 Hz, 1H), 3.24–3.32 (m, 1H), 3.37–3.44 (m, 1H), 3.51–3.61 (m, 2H), 4.53 (dd, J = 7.7 Hz, J = 13.8 Hz, 1H), 6.95–7.01 (m, 2H), 7.02–7.06 (m, 2H), 7.12–7.18 (m, 3H), 7.19–7.23 (m, 3H), 7.32–7.36 (m, 1H), 7.69 (d, J = 8.5 Hz, 2H), 7.79 (d, J = 8.5 Hz, 2H). 13C-NMR (CD3OD/CDCl3): δ 23.7, 24.2, 35.8, 37.8, 49.1, 51.9, 52.5, 55.8, 115.2, 117.9, 126.1, 126.8, 126.9, 127.6, 128.1, 128.6, 128.7, 128.9, 129.2, 130.1, 132.2, 136.6, 137.3, 166.2, 172.6. HRMS calcd. for (M + H)+: C29H31N4O2 467.2447, found 467.2431.
2-[(4-Cyanobenzoyl)amino]-N-[(3,4-dihydroisoquinolin-2(1H)-yl)propyl]-4-pentenamide (2b): This product was obtained from resin 23, allyl bromide, 4-cyanobenzoic acid, and tetrahydroisoquinoline as a light yellow oil (4 mg, 19% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (92:8). Initial LC/MS purity 60%, tR = 5.4 min; 1H-NMR (CD3OD/CDCl3): δ 1.97–2.04 (m, 2H), 2.49–2.58 (m, 1H), 2.60–2.68 (m, 1H), 3.06–3.11 (m, 3H), 3.24–3.31 (m, 1H), 3.32–3.38 (m, 1H), 3.40–3.48 (m, 2H), 4.03–4.19 (m, 2H), 4.29 (s, 1H), 4.61 (dd, J = 6.0 Hz, J = 7.7 Hz, 1H), 5.05–5.21 (m, 2H), 5.65–5.82 (m, 1H), 7.06 (d, J = 7.5 Hz, 1H), 7.08–7.14 (m, 1H), 7.18 (d, J = 7.1 Hz, 1H), 7.21–7.28 (m, 3H), 7.70 (d, J = 8.3 Hz, 2H), 7.97 (d, J = 8.5 Hz, 2H). 13C-NMR (CD3OD/CDCl3): δ 24.2, 25.3, 36.4, 36.7, 41.5, 44.4, 53.5, 53.8, 115.3, 118.0, 118.9, 126.6, 126.7, 127.2, 127.3, 128.1, 128.9, 129.0, 132.3, 132.8, 137.4, 166.1, 172.3. HRMS calcd. for (M + H)+: C25H29N4O2 417.2212, found 417.2200.
2-[(4-Cyanobenzoyl)amino]-N-[(3,4-dihydroisoquinolin-2(1H)-yl)propyl]decanamide (2c): Product 2c was obtained from resin 23, 1-iodooctane, 4-cyanobenzoic acid, and tetrahydroisoquinoline as a light yellow oil (7 mg, 29% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (95:5). Initial LC/MS purity 65%, tR = 11.8 min; 1H-NMR (CD3OD/CDCl3): δ 0.86 (t, J = 7.0 Hz, 3H), 1.17–1.48 (m, 12H), 1.71–1.95 (m, 2H), 1.99–21.6 (m, 2H), 2.99–3.52 (m, 7H), 3.55–3.79 (m, 1H), 3.92–4.25 (m, 1H), 4.30–4.69 (m, 2H), 7.03-7.15 (m, 1H), 7.20 (d, J = 7.1 Hz, 1H), 7.24–7.34 (m, 3H), 7.71 (d, J = 8.5 Hz, 2H), 8.00 (d, J = 8.7 Hz, 2H). 13C-NMR (CD3OD/CDCl3): δ 14.0, 22.6, 23.8, 24.3, 25.9, 29.1, 29.2, 29.3, 31.7, 32.0, 35.7, 49.3, 52.2, 52.6, 55.0, 115.2, 118.0, 126.1, 126.8, 127.6, 128.2, 128.7, 128.9, 130.1, 132.2, 137.4, 166.5, 171.1. HRMS calcd. for (M + H)+: C30H41N4O2 489.3151, found 489.3138.
α -[(4-Cyanobenzoyl)amino]-4-[(ethoxyhydroxyphosphinyl)difluoromethyl]-N-[(3,4-dihydroisoquinolin-2(1H)-yl)propyl]benzenepropanamide (2d): Product 2d was obtained from resin 23, diethyl [(4-α-bromomethyl)phenyldifluoromethyl]-phosphonate, 4-cyanobenzoic acid, and tetrahydroisoquinoline as a light yellow oil (6 mg, 19% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (92:8). Initial LC/MS purity 47%, tR = 5.9 min; 1H-NMR (CD3OD/CDCl3): δ 1.05–1.19 (m, 3H), 1.30–1.41 (m, 2H), 2.57–2.80 (m, 3H), 3.03–3.43 (m, 10H), 4.04–4.16 (1H), 4.25 (s, 1H), 4.70–4.83 (m, 1H), 7.08–7.36 (m, 8H), 7.56 (d, J = 7.3 Hz, 2H), 7.79 (d, J = 8.0 Hz, 2H), 8.01 (d, J = 8.2 Hz, 2H). 13C-NMR (125 MHz, CD3OD/CDCl3): δ 16.3, 22.9, 24.7, 36.1, 38.9, 41.6, 52.6, 52.8, 55.5, 62.6, 115.1, 117.9, 126.5, 126.7, 126.9, 127.1, 127.7, 128.0, 128.2, 128.5, 128.7, 128.9, 130.5, 132.2, 137.7, 165.6, 170.8. HRMS calcd. for (M + H)+: C32H37F2N4O5P 625.2313, found 625.2326.
Direct Comparison of Activation of Alcohol Resin 26 via Mesylation (to 27a) with that via Iodination (to 27b) using Optimal N-Alkylation and Cleavage Conditions: The mesylation of the alcohol resin 26 was the same as described above. Iodination of the resin 26 was performed as follows. After the free alcohol resin 26 was swelled in CH2Cl2 for 1 h and washed with DMF (3 × 1 mL), a pre-mixed solution of iodine (63 mg, 0.25 mmol, 5 equiv), PPh3 (66 mg, 0.25 mmol, 5 equiv), and imidazole (17 mg, 0.25 mmol, 5 equiv) in DMF (1.0 mL) was added. After 18 h, the filtered resin was washed with DMF (3 × 1 mL), MeOH (3 × 1 mL), DMF (2 × 1 mL), and CH2Cl2 (3 × 1 mL). After mesylated resin 27a and iodinated resin 27b were swelled in CH2Cl2 for 40 min, to each resin was added a solution of tetrahydroisoquinoline in DMSO (1 M, 0.75 mL, 0.75 mmol, 15 equiv). The reaction was heated to 80 °C over 3 h for mesylated resin or 50 °C over 6 h for iodinated resin. The resulting resin products were drained, sequentially washed with DMF (2 × 1 mL), MeOH (2 × 1 mL), DMF (3 × 1 mL), CH2Cl2 (4 × 1 mL), and cleaved with 90% TFA/CH2Cl2 (1 mL) over 1.5 h. The filtrate of the reaction mixture was collected and combined with washes of 50% TFA/CH2Cl2 (1 × 1 mL) and CH2Cl2 (1 × 1 mL) of the resin. A 100 μL sample of the combined solution was analyzed for crude purity by LC/MS. The crude purity for the product 2 was found to be 65% for the product through the mesylation and 88% for the product through the iodination process.
Synthesis of 32 New Resin Products 25 (Targeting Piperazine Derivatives 3 ) from the Same Aldimine of Glycine on BAL Resin 23 through Alkylation, Hydrolysis, and Acylation: Resin-bound Schiff base 23 (1.65 mmol) pre-swelled in CH2Cl2 for 1 h was evenly distributed to 33 of the reaction vessels in two separate 24-pack BillBoards via an isopycnic solution in CH2Cl2–NMP (9:5, v/v). 32 of the reaction vessels were arranged as two 4 × 4 grids on the BillBoards, and the 33rd reaction vessel was put at position on A5 on one of the BillBoards for the quality control experiment for the resin 23. After the drained resin (50 μmol) was washed with NMP (4 × 1 mL), to the four reaction vessels down the first column positions (i.e. A1, B1, C1 and D1) on both BillBoards were added 1-iodohexane (2.0 M, 0.25 mL, 0.50 mmol, 10 equiv). To the four reaction vessels down the 2nd column positions on both BillBoards were added 4-methoxybenzyl chloride (2.0 M, 0.25 mL, 0.50 mmol, 10 equiv). To the four reaction vessels down the 3rd column positions on both BillBoards were added 3-fluorobenzyl chloride (2.0 M, 0.25 mL, 0.50 mmol, 10 equiv). To the four reaction vessels down the 4th column positions on both BillBoards were added 4-fluorobenzyl bromide (2.0 M, 0.25 mL, 0.50 mmol, 10 equiv). To the control reaction vessel was added a solution of BnBr in NMP (2.0 M, 0.25 mL, 0.50 mmol, 10 equiv) for quality control of the starting resin 23.
Then a solution of BTPP in NMP (2.0 M, 0.25 mL, 0.50 mmol, 10 equiv) was added to each of the 33 reaction vessels. Alkylation was allowed to proceed at ambient temperature for 24 h with rotation. The alkylated resin product was filtered and washed with NMP (4 × 1 mL), CH
2Cl
2 (4 × 1 mL) and THF (3 × 1 mL). The resin was then treated with 1N HCl–THF (1:2, 1 mL) for 20 min. The resulting resin was filtered and washed with THF (3 × 1 mL), 10% DIEA/CH
2Cl
2 (5 × 1 mL), CH
2Cl
2 (2 × 1 mL). After the resin was swelled in CH
2Cl
2 (1 mL) for 1 h, and washed with DMF (3 × 1 mL), to the resins across row A positions (i.e. A1, A2, A3, A4) on both BillBoards were added a solution of cyclopropanecarboxylic acid and HOBt in DMF (0.38 M, 0.66 mL, 0.25 mmol, 5 equiv) which was pre-mixed (6 - 10 min before addition) with DIPCDI (32 mg, 0.25 mmol, 5 equiv). To the resins across row B positions on both BillBoards were added a solution of cyclohexanecarboxylic acid and HOBt in DMF (0.38 M, 0.66 mL, 0.25 mmol, 5 equiv) which was pre-mixed (6 - 10 min before addition) with DIPCDI (32 mg, 0.25 mmol, 5 equiv). To the resins across row C positions on both BillBoards were added a solution of benzoic acid and HOBt in DMF (0.38 M, 0.66 mL, 0.25 mmol, 5 equiv) which was pre-mixed (6 - 10 min before addition) with DIPCDI (32 mg, 0.25 mmol, 5 equiv). To the resins across row D positions on both BillBoards were added a solution of quinaldic acid and HOBt in DMF (0.38 M, 0.66 mL, 0.25 mmol, 5 equiv) which was pre-mixed (6 min before addition) with DIPCDI (32 mg, 0.25 mmol, 5 equiv; and it was observed that the colorless clear solution turned purple 2 min after DIPCDI was added). To the quality control reaction vial was added a solution of benzoic acid and HOBt in DMF (0.38 M, 0.66 mL, 0.25 mmol, 5 equiv) which was pre-mixed (6 - 10 min before addition) with DIPCDI (neat, 32 mg, 0.25 mmol, 5 equiv). The reaction mixture was rotated for 18 h. The completion of the reaction was confirmed by a negative chloranil test [
15], and the filtered resin product
25 was washed with DMF (4 × 1 mL), THF (3 × 1 mL), CH
2Cl
2 (3 × 1 mL), and dried in air.
Deprotection of the Silyl Ether on Resin 25 with TBAF and Subsequent Iodination using Triphenylphosphine, Iodine and Imidazole to Form Resin-Bound 27b: The resin product 25 was swelled in CH2Cl2 for 40 min, and the drained resin was washed with THF (3 × 1 mL). To the resin was added TBAF/THF (1 M, 1.0 mL, 1.0 mmol, 20 equiv), and the reaction was allowed to proceed for 18 h. The drained resin was washed with THF (3 × 1 mL), and CH2Cl2 (3 × 1 mL), and air dried. After this alcohol resin was swelled in CH2Cl2 for 1 h and washed with DMF (3 × 1 mL), a pre-mixed solution of iodine (63 mg, 0.25 mmol, 5 equiv), PPh3 (66 mg, 0.25 mmol, 5 equiv), and imidazole (17 mg, 0.25 mmol, 5 equiv) in DMF (1.0 mL) was added. After 18 h, the filtered resin was washed with DMF (3 × 1 mL), MeOH (3 × 1 mL), DMF (2 × 1 mL), and CH2Cl2 (3 × 1 mL).
Displacement of Iodo-Resin 27b with Amines and Subsequent Cleavage to Target Molecules 3: The air-dried resin 27b was swelled in CH2Cl2 for 40 min, and to the first 16 reaction vessels on one BillBoard were added a solution of 2-methoxyphenyl piperazine in anhydrous DMSO (1 M, 0.75 mL, 0.75 mmol, 15 equiv), while to the other 16 reaction vessels on the other BillBoard were added a solution of 3-chlorophenyl piperazine in anhydrous DMSO (1 M, 0.75 mL, 0.75 mmol, 15 equiv), and to the resin for quality control was added a tetrahydroisoquinoline solution in DMSO (1M, 0.75 mL, 0.75 mmol, 15 equiv). All reaction vessels were heated to 50 ◦C for 6 h with occasional agitation. The resulting resin product was drained, sequentially washed with DMF (2 × 1 mL), MeOH (2 × 1 mL), DMF (3 × 1 mL), CH2Cl2 (4 × 1 mL), and cleaved with 90% TFA/CH2Cl2 (1 mL) over 1.5 h, affording the target compound 3.
2-[(Cyclopropanecarbonyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-octanamide (3a): Product 3a was obtained from resin 23, 1-iodohexane, cyclopropanecarboxylic acid, and 2-methoxy-phenylpiperazine as an amorphous light yellow solid (10.5 mg, 46% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (92:8). Initial LC/MS purity 81%, tR = 8.2 min; 1H-NMR (CDCl3): δ 0.71–0.78 (m, 2H), 0.86 (t, J = 6.9 Hz, 3H), 0.89–0.96 (m, 2H), 1.19–1.38 (m, 10H), 1.42–1.51 (m, 1H), 1.57–1.69 (m, 1H), 1.79–1.90 (m, 3H), 2.74–2.83 (m, 2H), 2.89–3.02 (m, 3H), 3.18–3.28 (m, 3H), 3.33–3.43 (m, 2H), 3.87 (s, 3H), 4.36 (dd, J = 7.8 Hz, J = 13.7 Hz, 1H), 6.60 (d, J = 7.1 Hz, 1H), 6.88 (d, J = 7.7 Hz, 1H), 6.91–6.97 (m, 2H), 7.01–7.09 (m, 2H), 7.52–7.65 (m, 1H). 13C-NMR (CDCl3): δ 7.3, 7.4, 14.0, 14.6, 22.6, 24.4, 25.7, 29.0, 31.7, 32.8, 37.7, 49.3, 53.0, 53.8, 55.4, 55.9, 111.3, 118.6, 121.2, 123.8, 140.1, 152.2, 172.7, 173.8. HRMS calcd. for (M + H)+: C26H42N4O3 459.3335, found 459.3313.
2-[(Cyclopropanecarbonyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3-(4-methoxy-phenyl)propanamide (3b): Product 3b was obtained from resin 23, 4-methoxybenzyl chloride, cyclopropanecarboxylic acid, and 2-methoxyphenylpiperazine as light yellow oil (12.2 mg, 49% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 78%, tR = 6.1 min; 1H-NMR (CDCl3): δ 0.67–0.77 (m, 2H), 0.84–0.95 (m, 2H), 1.43–1.53 (m, 1H), 1.78–1.90 (m, 2H), 1.97–2.07 (m, 1H), 2.37 (t, J = 8.1 Hz, 1H), 2.68–2.79 (m, 2H), 2.84 (s, 1H), 2.98–3.05 (m, 2H), 3.20–3.29 (m, 4H), 3.33–3.42 (m, 2H), 3.76 (s, 3H), 3.86 (s, 3H), 4.50–4.79 (m, 1H), 6.81 (d, J = 8.6 Hz, 2H), 6.86 (d, J = 8.5 Hz, 2H), 6.91–6.95 (m, 2H), 7.03–7.08 (m, 1H), 7.14 (d, J = 8.5 Hz, 2H), 7.46 (brs, 1H). 13C-NMR (CDCl3): δ 7.4, 7.5, 14.6, 17.7, 23.9, 29.6, 30.7, 37.6, 44.0, 48.7, 49.5, 52.8, 55.2, 55.5, 111.3, 113.9, 118.7, 121.2, 123.9, 128.8, 130.5, 139.7, 152.2, 158.5, 171.9, 174.0. HRMS calcd. for (M + H)+: C28H39N4O4 495.2907, found 495.2914.
2-[(Cyclopropanecarbonyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3-(3-fluoro-phenyl)propanamide (3c): Product 3c was obtained from resin 23, 3-fluorobenzyl chloride, cyclopropanecarboxylic acid, and 2-methoxyphenylpiperazine as light yellow oil (4.6 mg, 19% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 65%, tR = 6.5 min; 1H-NMR (CDCl3): δ 0.66–0.82 (m, 2H), 0.85–0.99 (m, 2H), 1.32–1.49 (m, 1H), 1.61–1.81 (m, 2H), 2.48–2.64 (m, 2H), 2.65–2.87 (m, 3H), 3.03–3.22 (m, 5H), 3.24–3.47 (m, 3H), 3.87 (s, 3H), 4.49–4.69 (m, 1H), 6.53 (brs, 1H), 6.87 (d, J = 8.1 Hz, 1H), 6.90–6.97 (m, 4H), 6.98–7.06 (m, 2H), 7.21–7.25 (m, 1H), 7.34 (brs, 1H). 13C-NMR (CDCl3): δ 7.4, 7.5, 14.7, 24.4, 31.8, 38.7, 50.1, 53.2, 53.3, 54.9, 55.4, 77.6, 111.2 113.7, 116.4, 118.4, 121.1, 123.4, 125.1, 129.9, 139.4, 140.6, 152.2, 161.8, 163.8, 170.7, 173.5. HRMS calcd. for (M + H)+: C27H36FN4O3 483.2771, found 483.2766.
2-[(Cyclopropanecarbonyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3-(4-fluoro-phenyl)propanamide (3d): Product 3d was obtained from resin 23, 4-fluorobenzyl bromide, cyclopropanecarboxylic acid, and 2-methoxyphenylpiperazine as light yellow oil (18.3 mg, 76% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 83%, tR = 6.4 min; 1H-NMR (CDCl3): δ 0.66–0.77 (m, 2H), 0.78–0.94 (m, 2H), 1.42–1.55 (m, 1H), 1.81–1.99 (m, 2H), 2.79–2.93 (m, 2H), 3.02 (dd, J = 7.4 Hz, J = 13.8 Hz, 2H), 3.09 (dd, J = 6.5 Hz, J = 13.8 Hz, 2H), 3.22–3.43 (m, 6H), 3.87 (s, 3H), 4.68 (dd, J = 7.3 Hz, J = 14.6 Hz, 1H), 6.89 (d, J = 8.4 Hz, 1H), 6.91–7.02 (m, 5H), 7.04–7.09 (m, 1H), 7.16–7.24 (m, 2H), 7.62 (brs, 1H). 13C-NMR (CDCl3): δ 7.4, 7.5, 14.7, 23.8, 36.5, 37.5, 48.1, 52.6, 54.8, 55.0, 55.4, 111.4, 115.2 115.4, 118.7, 121.2, 124.3, 130.9, 132.6, 139.2, 152.2, 160.9, 162.8, 172.1, 174.2. HRMS calcd. for (M + H)+: C27H36FN4O3 483.2771, found 483.2755.
2-[(Cyclohexanecarbonyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}octanamide (3e): Product 3e was obtained from resin 23, 1-iodohexane, cyclohexanecarboxylic acid, and 2-methoxy-phenylpiperazine as light yellow oil (12.8 mg, 51% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 81%, tR = 10.8 min; 1H- NMR (CDCl3): δ 0.85 (t, J = 6.8 Hz, 3H), 1.20–1.30 (m, 11H), 1.36–1.48 (m, 2H), 1.58–1.68 (m, 2H), 1.73–1.88 (m, 7H), 1.97–2.07 (m, 0.5H), 2.08–2.21 (m, 1H), 2.38 (t, J = 8.1 Hz, 0.5H), 2.65–2.79 (m, 2H), 2.82–2.96 (m, 3H), 3.13–3.26 (m, 3H), 3.29–3.48 (m, 3H), 3.88 (s, 3H), 4.35 (dd, J = 7.6 Hz, J = 13.9 Hz, 1H), 6.38 (brs, 1H), 6.87 (d, J = 8.1 Hz, 1H), 6.89–6.96 (m, 2H), 6.99–7.06 (m, 1H), 7.64 (brs, 1H). 13C-NMR (CDCl3): δ 14.0, 17.7, 22.5, 24.6, 25.6, 25.7, 29.0, 29.3, 29.8, 30.7, 31.6, 32.8, 38.2, 45.3, 49.7, 53.2, 55.4, 111.3, 118.5, 121.1, 123.5, 140.4, 152.2, 172.5, 176.5. HRMS calcd. for (M + H)+: C29H49N4O3 501.3805, found 501.3811.
2-[(Cyclohexanecarbonyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3(4-methoxy-phenyl)propanamide (3f): Product 3f was obtained from resin 23, 4-methoxybenzyl chloride, cyclo-hexanecarboxylic acid, and 2-methoxyphenylpiperazine as light yellow oil (9.9 mg, 37% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 65%, tR = 8.7 min; 1H-NMR (CDCl3): δ 1.16–1.27 (m, 3H), 1.32–1.43 (m, 2H), 1.61–1.69 (m, 3H), 1.72–1.83 (m, 4H), 1.97–2.04 (m, 1H), 2.05–2.13 (m, 1H), 2.38 (t, J = 8.1 Hz, 1H), 2.45–2.52 (m, 2H), 2.62–2.66 (m, 1H), 2.68–2.73 (m, 1H), 2.84 (s, 1H), 2.97 (d, J = 7.1 Hz, 2H), 3.06–3.13 (m, 2H), 3.22–3.34 (m, 2H), 3.38 (t, J = 7.1 Hz, 1H), 3.76 (s, 3H), 3.86 (s, 3H), 4.50 (dd, J = 7.2 Hz, J = 14.6 Hz, 1H), 6.30 (d, J = 7.2 Hz, 1H), 6.80 (d, J = 8.6 Hz, 2H), 6.87 (d, J = 7.9 Hz, 1H), 6.94 (d, J = 4.2 Hz, 2H), 6.99–7.05 (m, 1H), 7.11 (d, J = 8.6 Hz, 2H), 7.19 (brs, 1H). 13C-NMR (CDCl3): δ 17.7, 25.6, 25.7, 29.3, 29.7, 30.7, 38.1, 45.2, 49.5, 53.2, 54.7, 55.2, 55.4, 56.6, 111.2, 114.1, 118.4, 121.1, 123.3, 128.8, 130.4, 140.7, 152.2, 158.5, 171.1, 175.9. HRMS calcd. for (M + H)+: C31H45N4O4 537.3441, found 537.3439.
2-[(Cyclohexanecarbonyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3-(3-fluoro-phenyl)propanamide (3g): Product 3g was obtained from resin 23, 3-fluorobenzyl chloride, cyclo-hexanecarboxylic acid, and 2-methoxyphenylpiperazine as yellow oil (13.9 mg, 53% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 65%, tR = 9.1 min; 1H-NMR (CDCl3): δ 1.14–1.37 (m, 5H), 1.60–1.78 (m, 5H), 1.93–2.07 (m, 3H), 2.08-2.18 (m, 1H), 2.37 (t, J = 8.2 Hz, 1H), 2.84 (s, 1H), 2.94–3.05 (m, 3H), 3.16 (dd, J = 6.0 Hz, J = 13.9 Hz, 2H), 3.27–3.34 (m, 3H), 3.35–3.44 (m, 3H), 3.87 (s, 3H), 4.66 (dd, J = 7.9 Hz, J = 14.1 Hz, 1H), 6.60 (d, J = 7.8 Hz, 1H), 6.86–6.97 (m, 5H), 7.00 (d, J = 7.7 Hz, 1H), 7.05–7.11 (m, 1H), 7.21–7.25 (m, 1H), 7.60–7.70 (m, 1H). 13C-NMR (CDCl3): δ 17.7, 23.7, 25.7, 29.1, 29.7, 30.7, 37.7, 44.9, 47.7, 49.5, 52.5, 54.4, 55.5, 111.4, 113.7, 116.3, 118.9, 121.2, 124.5, 125.1, 130.0, 138.9, 139.5, 152.1, 161.8, 163.8, 172.3, 176.8. HRMS calcd. for (M + H)+: C30H42FN4O3 525.3241, found 525.3222.
2-[(Cyclohexanecarbonyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3-(4-fluoro-phenyl)propanamide (3h): Product 3h was obtained from resin 23, 4-fluorobenzyl bromide, cyclo-hexanecarboxylic acid, and 2-methoxyphenylpiperazine as an amorphous light yellow solid (16.3 mg, 62% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 65%, tR = 9.0 min; 1H-NMR (CDCl3): δ 1.13-1.27 (m, 3H), 1.28-1.41 (m, 2H), 1.59-1.66 (m, 1H), 1.67–1.82 (m, 6H), 1.98–2.05 (m, 0.4H), 2.05–2.14 (m, 1H), 2.37 (t, J = 8.2 Hz, 0.4H), 2.54–2.66 (m, 2H), 2.75–2.86 (m, 3H), 2.93–3.00 (m, 1H), 3.02–3.08 (m, 1H), 3.09–3.20 (m, 3H), 3.22–3.41 (m, 3H), 3.86 (s, 3H), 4.58 (dd, J = 7.3 Hz, J = 14.8 Hz, 1H), 6.48 (brs, 1H), 6.87 (d, J = 8.1 Hz, 1H), 6.91–6.99 (m, 4H), 7.00–7.07 (m, 1H), 7.12–7.20 (m, 2H), 7.49 (brs, 1H). 13C-NMR (CDCl3): δ 17.6, 24.4, 25.7, 29.2, 29.7, 30.7, 37.9, 45.1, 49.5, 53.1, 54.5, 55.4, 56.2, 111.3, 115.4, 118.5, 121.1, 123.6, 130.9, 132.5, 140.3, 152.2, 160.9, 162.8, 171.3, 176.3. HRMS calcd. for (M + H)+: C30H42FN4O3 525.3241, found 525.3244.
2-[(Benzoyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}octanamide (3i): Product 3i was obtained from resin 23, 1-iodohexane, benzoic acid, and 2-methoxyphenylpiperazine as light yellow oil (17.4 mg, 70% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 83%, tR = 10.1 min; 1H-NMR (CDCl3): δ 0.81–0.89 (m, 3H), 1.21–1.41 (m, 8H), 1.71–1.83 (m, 1H), 1.84–1.98 (m, 3H), 1.99–2.06 (m, 0.4H), 2.35 (t, J = 8.2 Hz, 0.5H), 2.83 (s, 1H), 2.90–2.99 (m, 2H), 3.02–3.13 (m, 2H), 3.19–3.29 (m, 3H), 3.31–3.49 (m, 3H), 3.85 (s, 3H), 4.57 (dd, J = 7.7 Hz, J = 13.7 Hz, 1H), 6.82–6.97 (m, 3H), 7.01–7.10 (m, 1H), 7.29–7.36 (m, 1H), 7.40 (t, J = 7.5 Hz, 2H), 7.48 (t, J = 7.4 Hz, 1H), 7.86 (d, J = 7.3 Hz, 2H), 7.94 (brs, 1H). 13C-NMR (CDCl3): δ 14.0, 17.7, 22.5, 23.9, 25.8, 29.0, 30.7, 31.6, 32.6, 37.0, 48.4, 49.5, 52.8, 54.5, 55.4, 111.3, 118.7, 121.2, 124.1, 127.3, 128.6, 131.8, 133.6, 139.5, 152.2, 167.6, 173.1. HRMS calcd. for (M + H)+: C29H43N4O3 495.3335, found 495.3337.
2-[(Benzoyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3(4-methoxyphenyl)-propan-amide (3j): Product 3j was obtained from resin 23, 4-methoxybenzyl chloride, benzoic acid, and 2-methoxyphenylpiperazine as an amorphous light yellow solid (10.3 mg, 39% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 71%, tR = 7.9 min; 1H-NMR (CDCl3): δ 1.72-1.87 (m, 2H), 1.96–2.09 (m, 1H), 2.37 (t, J = 8.2 Hz, 1H), 2.63–2.73 (m, 2H), 2.79–2.91 (m, 3H), 3.10–3.20 (m, 5H), 3.31–3.39 (m, 2H), 3.76 (s, 3H), 3.86 (s, 3H), 4.74 (dd, J = 7.2 Hz, J = 14.4 Hz, 1H), 6.83 (d, J = 8.6 Hz, 2H), 6.86–6.89 (m, 1H), 6.89–6.97 (m, 2H), 7.02–7.09 (m, 2H), 7.18 (d, J = 8.6 Hz, 2H), 7.37–7.45 (m, 3H), 7.46–7.51 (m, 1H), 7.74–7.79 (m, 2H). 13C-NMR (CDCl3): δ 17.7, 24.2, 29.6, 30.7, 37.9, 49.2, 49.4, 52.9, 55.2, 55.4, 55.6, 111.3, 114.1, 118.6, 121.2, 123.7, 127.1, 128.6, 128.7, 130.4, 131.7, 133.8, 152.2, 158.6, 167.1, 171.4. HRMS calcd. for (M + H)+: C31H29N4O4 531.2971, found 531.2951.
2-[(Benzoyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3-(3-fluorophenyl)-propanamide (3k): Product 3k was obtained from resin 23, 3-fluorobenzyl chloride, benzoic acid, and 2-methoxyphenylpiperazine as yellow oil (12.6 mg, 49% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 75%, tR = 8.4 min; 1H- NMR (CDCl3): δ 1.76–1.93 (m, 2H), 1.96–2.08 (m, 1H), 2.37 (t, J = 8.2 Hz, 1H), 2.71–2.82 (m, 2H), 2.83–2.86 (m, 1H), 2.87–2.97 (m, 2H), 3.14–3.25 (m, 5H), 3.28–3.34 (m, 1H), 3.36–3.40 (m, 1H), 3.86 (s, 3H), 4.81 (dd, J = 7.2 Hz, J = 14.6 Hz, 1H), 6.86–6.89 (m, 1H), 6.89–6.96 (m, 3H), 6.97–7.01 (m, 1H), 7.02–7.08 (m, 2H), 7.16–7.22 (m, 1H), 7.23–7.26 (m, 1H), 7.37–7.43 (m, 2H), 7.45–7.51 (m, 1H), 7.59–7.70 (m, 1H), 7.73–7.80 (m, 2H). 13C-NMR (CDCl3): δ 17.7, 24.0, 29.6, 30.7, 38.3, 48.9, 49.4, 52.9, 55.3, 55.4, 111.3, 113.8, 113.9, 118.7, 121.2, 123.9, 125.1, 127.2, 128.6, 130.1, 131.8, 133.6, 139.4, 139.5, 152.2, 161.9, 163.8, 167.2, 171.3. HRMS calcd. for (M + H)+: C30H36FN4O3 519.2771, found 519.2782.
2-[(Benzoyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3-(4-fluorophenyl)-propanamide (3l): Product 3l was obtained from resin 23, 4-fluorobenzyl bromide, benzoic acid, and 2-methoxy-phenylpiperazine as brownish oil (19.7 mg, 76% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (94:6). Initial LC/MS purity 86%, tR = 8.3 min; 1H-NMR (CDCl3): δ 1.78–1.93 (m, 2H), 1.95–2.06 (m, 0.5H), 2.36 (t, J = 8.2 Hz, 0.5H), 2.75–2.88 (m, 3H), 2.93–3.04 (m, 2H), 3.07–3.16 (m, 2H), 3.17–3.32 (m, 5H), 3.34–3.41 (m, 1H), 3.85 (s, 3H), 4.71–4.92 (m, 1H), 6.85–6.91 (m, 2H), 7.01–7.09 (m, 1H), 7.19–7.25 (m, 2H), 7.37 (t, J = 7.6 Hz, 2H), 7.46 (t, J = 7.4 Hz, 1H), 7.77 (d, J = 7.4 Hz, 2H), 7.85 (brs, 1H). 13C-NMR (CDCl3): δ 17.6, 23.8, 29.6, 30.7, 37.5, 48.5, 49.5, 52.7, 55.4, 55.5, 111.3, 115.5, 118.7, 121.2, 124.1, 127.2, 128.5, 130.9, 131.9, 132.5, 133.4, 139.5, 152.2, 160.9, 162.8, 167.5, 171.8. HRMS calcd. for (M + H)+: C30H36FN4O3 519.2771, found 519.2745.
2-[(Quinolin-2-oyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}octanamide (3m): Product 3m was obtained from resin 23, 1-iodohexane, quinaldic acid, and 2-methoxyphenylpiperazine as brownish oil (18.4 mg, 67% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (95:5). Initial LC/MS purity 80%, tR = 12.1 min; 1H-NMR (CDCl3): δ 0.85 (t, J = 6.9 Hz, 3H), 1.24–1.30 (m, 4H), 1.32–1.49 (m, 4H), 1.78–1.92 (m, 1H), 1.94–2.10 (m, 3.5H), 2.37 (t, J = 8.1 Hz, 0.5H), 2.83 (s, 1H), 2.96–3.10 (m, 3H), 3.19–3.32 (m, 4H), 3.34–3.42 (m, 2H), 3.43–3.53 (m, 1H), 3.84 (s, 3H), 4.59 (dd, J = 7.3 Hz, J = 13.7 Hz, 1H), 6.82–6.96 (m, 3H), 6.99–7.09 (m, 1H), 7.57–7.71 (m, 2H), 7.76 (t, J = 8.2 Hz, 1H), 7.84 (d, J = 8.2 Hz, 1H), 8.14 (d, J = 8.5 Hz, 1H), 8.21–8.34 (m, 2H), 8.61–8.82 (m, 1H). 13C-NMR (CDCl3): δ 14.0, 17.6, 22.5, 25.8, 28.9, 30.7, 31.6, 32.3, 48.2, 49.5, 52.7, 54.2, 55.4, 111.3, 118.8, 121.2, 124.1, 127.7, 128.1, 129.4, 129.9, 130.2, 137.6, 139.4, 146.5, 148.9, 152.1, 164.9, 172.4. HRMS calcd. for (M + H)+: C32H44N5O3 546.3444, found 546.3419.
2-[(Quinolin-2-oyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3-(4-methoxyphenyl)-propanamide (3n): Product 3n was obtained from resin 23, 4-methoxybenzyl chloride, quinaldic acid, and 2-methoxyphenylpiperazine as brownish oil (10.5 mg, 36% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (94:6). Initial LC/MS purity 66%, tR = 9.9 min; 1H-NMR (CDCl3): δ 1.61–1.87 (m, 2H), 1.95–2.08 (m, 1H), 2.37 (t, J = 8.1 Hz, 1H), 2.56–2.67 (m, 2H), 2.77–2.88 (m, 3H), 3.08–3.15 (m, 2H), 3.16–3.28 (m, 3H), 3.32–3.35 (m, 1H), 3.36–3.39 (m, 1H), 3.77 (s, 3H), 3.85 (s, 3H), 4.76 (dd, J = 7.7 Hz, J = 14.4 Hz, 1H), 6.81–6.88 (m, 4H), 6.89–6.93 (m, 1H), 6.98–7.05 (m, 1H), 7.15–7.22 (m, 1H), 7.23–7.26 (m, 2H), 7.57–7.66 (m, 1H), 7.71–7.80 (m, 1H), 7.86 (d, J = 8.1 Hz, 1H), 8.10 (d, J = 8.4 Hz, 1H), 8.21 (d, J = 8.5 Hz, 1H), 8.27 (d, J = 8.5 Hz, 1H), 8.78 (d, J = 8.1 Hz, 1H). 13C-NMR (CDCl3): δ 17.7, 24.4, 29.6, 30.7, 37.7, 49.4, 52.9, 55.2, 55.4, 55.5, 56.1, 111.2, 114.0, 118.7, 121.1, 123.5, 127.7, 128.1, 128.8, 129.4, 129.9, 130.2, 130.5, 137.5, 146.5, 149.1, 152.2, 158.6, 164.5, 170.9. HRMS calcd. for (M + H)+: C34H40N5O4 582.3080, found 582.3058.
2-[(Quinolin-2-oyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3-(3-fluorophenyl)-propanamide (3o): Product 3o was obtained from resin 23, 3-fluorobenzyl chloride, quinaldic acid, and 2-methoxyphenylpiperazine as brownish oil (15.3 mg, 54% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 77%, tR = 10.3 min; 1H- NMR (CDCl3): δ 1.88–2.12 (m, 3H), 2.37 (t, J = 8.1 Hz, 1H), 2.84 (s, 1H), 2.93–3.04 (m, 2H), 3.19–3.44 (m, 9H), 3.86 (s, 3H), 4.84 (dd, J = 7.0 Hz, J = 14.5 Hz, 1H), 6.85–6.98 (m, 4H), 7.02–7.10 (m, 2H), 7.13 (d, J = 7.7 Hz, 1H), 7.27-7.33 (m, 1H), 7.40–7.49 (m, 1H), 7.62 (t, J = 7.1 Hz, 1H), 7.73–7.81 (m, 1H), 7.85 (d, J = 8.1 Hz, 1H), 8.10 (d, J = 8.4 Hz, 1H), 8.17 (d, J = 8.5 Hz, 1H), 8.27 (d, J = 8.4 Hz, 1H), 8.76 (d, J = 7.8 Hz, 1H). 13C-NMR (CDCl3): δ 17.7, 23.7, 29.6, 30.7, 37.9, 47.8, 49.4, 52.5, 54.9, 55.4, 111.3, 114.1, 116.3, 118.5, 118.8, 121.2, 124.3, 125.2, 127.7, 128.1, 129.4, 129.9, 130.2, 137.5, 139.3, 146.5, 148.8, 152.1, 161.9, 162.7, 163.8, 164.8, 171.3. HRMS calcd. for (M + H)+: C33H37FN5O3 570.2880, found 570.2853.
2-[(Quinolin-2-oyl)amino]-N-{[4-(2-methoxyphenyl)-piperazin-1-yl]propyl}-3-(4-fluorophenyl)-propanamide (3p): Product 3p was obtained from resin 23, 4-fluorobenzyl bromide, quinaldic acid, and 2-methoxyphenylpiperazine as brownish oil (22.1 mg, 78% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 85%, tR = 10.2 min; 1H-NMR (CDCl3): δ 1.92–2.08 (m, 2.5H), 2.32–2.42 (m, 0.5H), 2.83 (s, 1H), 2.93–3.04 (m, 2H), 3.18–3.44 (m, 10H), 3.85 (s, 3H), 4.83 (dd, J = 7.3 Hz, J = 14.7 Hz, 1H), 6.84–6.95 (m, 3H), 6.96–7.02 (m, 2H), 7.03–7.09 (m, 1H), 7.28–7.36 (m, 2H), 7.47–7.56 (m, 1H), 7.61 (t, J = 7.4 Hz, 1H), 7.76 (t, J = 7.1 Hz, 1H), 7.85 (d, J = 8.2 Hz, 1H), 8.09 (d, J = 8.5 Hz, 1H), 8.17 (d, J = 8.5 Hz, 1H), 8.26 (d, J = 8.5 Hz, 1H), 8.74 (d, J = 7.8 Hz, 1H). 13C-NMR (CDCl3): δ 17.7, 23.6, 29.6, 30.7, 36.5, 37.5, 47.7, 49.4, 52.5, 54.6, 55.2, 55.4, 111.3, 115.4, 118.6, 118.9, 121.2, 124.3, 127.7, 128.1, 129.4, 129.9, 130.2, 131.0, 132.5, 139.1, 146.5, 148.9, 152.1, 160.9, 162.4, 162.9, 164.7, 171.5. HRMS calcd. for (M + H)+: C33H37FN5O3 570.2880, found 570.2870.
2-[(Cyclopropanecarbonyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}octanamide (3q): Product 3q was obtained from resin 23, 1-iodohexane, cyclopropanecarboxylic acid, and 3-chloro-phenylpiperazine as brownish oil (11.2 mg, 48% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 68%, tR = 9.7 min; 1H- NMR (CDCl3): δ 0.66–0.79 (m, 2H), 0.81–0.97 (m, 5H), 1.18–1.37 (m, 8.6H), 1.38–1.52 (m, 1H), 1.56–1.70 (m, 1H), 1.76–1.84 (m, 1H), 1.84–1.93 (m, 1.6H), 1.96–2.08 (m, 0.4H), 2.37 (t, J = 8.1 Hz, 0.4H), 2.72–2.83 (m, 2H), 2.84 (s, 1H), 2.87–3.01 (m, 3H), 3.27–3.44 (m, 6H), 4.34 (dd, J = 7.5 Hz, J = 14.1 Hz, 1H), 6.62 (brs, 1H), 6.75–6.81 (m, 1H), 6.83–6.90 (m, 2H), 7.15–7.22 (m, 1H), 7.39–7.57 (m, 1H). 13C-NMR (CDCl3): δ 7.4, 7.5, 14.0, 14.6, 17.7, 22.5, 24.5, 25.6, 29.0, 31.6, 32.6, 47.7, 49.5, 52.4, 53.9, 55.7, 114.4, 116.5, 120.5, 130.3, 135.1, 151.4, 172.7, 174.1. HRMS calcd. for (M + H)+: C25H40ClN4O2 463.2840, found 463.2841.
2-[(Cyclopropanecarbonyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}-3(4-methoxy-phenyl)propanamide (3r): Product 3r was obtained from resin 23, 4-methoxybenzyl chloride, cyclopropanecarboxylic acid, and 3-chlorophenyl piperazine as yellow oil (10.2 mg, 41% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (92:8). Initial LC/MS purity 60%, tR = 7.7 min; 1H-NMR (CDCl3): δ 0.67–0.79 (m, 2H), 0.85–0.94 (m, 2H), 1.39–1.44 (m, 1H), 1.71–1.83 (m, 2H), 1.97–2.08 (m, 1H), 2.37 (t, J = 8.2 Hz, 1H), 2.57–2.64 (m, 2H), 2.75–2.82 (m, 2H), 2.83–2.85 (m, 1H), 2.97–3.01 (m, 1H), 3.24–3.30 (m, 5H), 3.36–3.41 (m, 1H), 3.77 (s, 3H), 4.45–4.68 (m, 1H), 6.64 (brs, 1H), 6.77 (d, J = 7.4 Hz, 1H), 6.82 (d, J = 8.4 Hz, 2H), 6.87 (d, J = 7.0 Hz, 2H), 7.13 (d, J = 8.4 Hz, 2H), 7.18 (t, J = 8.3 Hz, 1H). 13C-NMR (CDCl3): δ 7.4, 7.5, 13.5, 14.6, 17.7, 29.6, 30.7, 37.7, 47.7, 49.5, 52.3, 55.3, 113.9, 114.4, 116.4, 120.4, 128.8, 130.3, 130.4, 135.1, 151.4, 158.5, 171.6, 173.8. HRMS calcd. for (M + H)+: C27H36ClN4O3 499.2476, found 499.2467.
2-[(Cyclopropanecarbonyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}-3-(3-fluorophenyl)-propanamide (3s): Product 3s was obtained from resin 23, 3-fluorobenzyl chloride, cyclo-propanecarboxylic acid, and 3-chlorophenylpiperazine as light yellow oil (8.0 mg, 33% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 57%, tR = 8.2 min; 1H-NMR (CDCl3): δ 0.67–0.81 (m, 2H), 0.86–0.96 (m, 2H), 1.37–1.47 (m, 1H), 1.77 (brs, 2H), 2.57–2.89 (m, 5H), 3.04–3.12 (m, 2H), 3.22–3.41 (m, 7H), 4.47–4.72 (m, 1H), 6.55 (brs, 1H), 6.74–6.81 (m, 1H), 6.85–6.89 (m, 2H), 6.91–6.96 (m, 2H), 7.00 (d, J = 7.7 Hz, 1H), 7.19 (t, J = 8.4 Hz, 2H), 7.23–7.26 (m, 1H). 13C-NMR (CDCl3): δ 7.5, 7.6, 14.6, 24.4, 38.3, 47.9, 52.5, 54.9, 56.0, 113.7, 114.3, 116.2, 116.4, 120.3, 125.2, 130.0, 130.2, 135.1, 139.5, 151.5, 161.8, 163.8, 171.4, 173.8. HRMS calcd. for (M + H)+: C26H33ClFN4O2 487.2276, found 487.2299.
2-[(Cyclopropanecarbonyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}-3-(4-fluorophenyl)-propanamide (3t): Product 3t was obtained from resin 23, 4-fluorobenzyl bromide, cyclo-propanecarboxylic acid, and 3-chlorophenylpiperazine as brown oil (9.9 mg, 41% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 73%, tR = 8.1 min; 1H-NMR (CDCl3): δ 0.67–0.78 (m, 2H), 0.83–0.94 (m, 2H), 1.37–1.47 (m, 1H), 1.69–1.85 (m, 2H), 2.58–2.73 (m, 2H), 2.75–2.94 (m, 4H), 2.98–3.07 (m, 2H), 3.21–3.39 (m, 6H), 4.58 (dd, J = 7.3 Hz, J = 14.7 Hz, 1H), 6.63 (brs, 1H), 6.74–6.79 (m, 1H), 6.84–6.91 (m, 2H), 6.94–7.01 (m, 2H), 7.10–7.26 (m, 4H). 13C-NMR (CDCl3): δ 7.4, 7.6, 14.6, 24.3, 37.7, 47.6, 52.3, 55.1, 55.6, 114.5, 115.4, 116.5, 120.6, 130.3, 130.9, 132.6, 135.1, 151.3, 160.9, 162.8, 171.4, 173.9. HRMS calcd. for (M + H)+: C26H33ClFN4O2 487.2276, found 487.2292.
2-[(Cyclohexanecarbonyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}octanamide (3u): Product 3u was obtained from resin 23, 1-iodohexane, cyclohexanecarboxylic acid, and 3-chlorophenyl piperazine as brown oil (10.9 mg, 43% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (94:6). Initial LC/MS purity 66%, tR = 12.2 min; 1H-NMR (CDCl3): δ 0.76–0.92 (m, 3H), 1.16–1.33 (m, 11H), 1.35–1.47 (m, 2H), 1.55–1.69 (m, 2H), 1.71-1.95 (m, 7H), 1.97–2.07 (m, 0.5H), 2.09–2.22 (m, 1H), 2.38 (t, J = 8.0 Hz, 0.5H), 2.78–2.86 (m, 2H), 2.89–3.04 (m, 3H), 3.25–3.45 (m, 6H), 4.15–4.44 (m, 1H), 6.38 (brs, 1H), 6.78 (d, J = 8.1 Hz, 1H), 6.82–6.93 (m, 2H), 7.18 (t, J = 8.3 Hz, 1H), 7.53 (brs, 1H). 13C-NMR (CDCl3): δ 14.0, 17.7, 22.5, 25.5, 25.7, 28.9, 29.3, 29.8, 31.6, 32.4, 37.3, 45.2, 47.6, 49.5, 52.4, 53.5, 55.5, 114.5, 116.6, 120.6, 130.3, 135.1, 151.3, 172.8, 176.7. HRMS calcd. for (M + H)+: C28H46ClN4O2 505.3309, found 505.3318.
2-[(Cyclohexanecarbonyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}-3(4-methoxyphenyl)-propanamide (3v): Product 3v was obtained from resin 23, 4-methoxybenzyl chloride, cyclo-hexanecarboxylic acid, and 3-chlorophenylpiperazine as an amorphous off-white solid (6.9 mg, 26% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 55%, tR = 10.1 min; 1H-NMR (CDCl3): δ 1.17–1.26 (m, 3H), 1.31–1.41 (m, 2H), 1.61–1.67 (m, 1H), 1.71–1.84 (m, 7H), 1.98–2.05 (m, 0.6H), 2.07–2.15 (m, 1H), 2.37 (t, J = 8.2 Hz, 0.6H), 2.59–2.71 (m, 3H), 2.84 (s, 1H), 2.98 (d, J = 6.7 Hz, 2H), 3.24–3.35 (m, 6H), 3.78 (s, 3H), 4.51 (dd, J = 7.1 Hz, J = 14.3 Hz, 1H), 6.22 (brs, 1H), 6.75–6.79 (m, 1H), 6.82 (d, J = 8.5 Hz, 2H), 6.86–6.89 (m, 2H), 6.96–7.07 (m, 1H), 7.12 (d, J = 8.6 Hz, 2H), 7.16–7.22 (m, 1H). 13C-NMR (CDCl3): δ 17.7, 24.4, 25.5, 25.7, 29.3, 29.7, 30.7, 37.7, 45.1, 49.4, 52.4, 54.8, 55.3, 113.9, 114.4, 116.4, 120.4, 128.8, 130.2, 130.4, 135.1, 151.4, 158.5, 171.5, 176.2. HRMS calcd. for (M + H)+: C30H42ClN4O3 541.2945, found 541.2943.
2-[(Cyclohexanecarbonyl)amino]-N-{[4-(3-chloroyphenyl)-piperazin-1-yl]propyl}-3-(3-fluorophenyl)-propanamide (3w): Product 3w was obtained from resin 23, 3-fluorobenzyl chloride, cyclo-hexanecarboxylic acid, and 3-chlorophenylpiperazine as an amorphous light yellow solid (8.5 mg, 32% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (94:6). Initial LC/MS purity 54%, tR = 10.6 min; 1H-NMR (CDCl3): δ 1.17–1.28 (m, 3H), 1.29–1.41 (m, 2H), 1.62–1.81 (m, 7H), 1.99–2.14 (m, 2H), 2.37 (t, J = 8.0 Hz, 1H), 2.49–2.59 (m, 2H), 2.62–2.73 (m, 3H), 2.84 (s, 1H), 2.96–3.09 (m, 2H), 3.18–3.25 (m, 3H), 3.27–3.31 (m, 1H), 3.38 (t, J = 7.0 Hz, 1H), 4.48–4.64 (m, 1H), 6.32 (brs, 1H), 6.77 (d, J = 8.2 Hz, 1H), 6.83–6.89 (m, 2H), 6.90–6.96 (m, 2H), 6.98 (d, J = 7.6 Hz, 1H), 7.18 (t, J = 8.0 Hz, 1H), 7.21–7.26 (m, 1H). 13C-NMR (CDCl3): δ 17.7, 24.7, 25.5, 25.6, 29.3, 29.7, 30.7, 38.3, 45.1, 48.1, 49.5, 52.6, 54.4, 56.2, 113.8, 113.9, 114.2, 116.2, 116.3, 120.0, 125.1, 130.2, 139.3, 151.7, 161.8, 163.8, 170.9, 176.2. HRMS calcd. for (M + H)+: C29H39ClFN4O2 529.2746, found 529.2736.
2-[(Cyclohexanecarbonyl)amino]-N-{[4-(3-chloroyphenyl)-piperazin-1-yl]propyl}-3-(4-fluorophenyl)-propanamide (3x): Product 3x was obtained from resin 23, 4-fluorobenzyl bromide, cyclo-hexanecarboxylic acid, and 3-chlorophenylpiperazine as an amorphous yellow solid (11.7 mg, 44% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 72%, tR = 10.5 min; 1H-NMR (CDCl3): δ 1.11–1.28 (m, 3H), 1.29–1.41 (m, 2H), 1.60–1.67 (m, 1H), 1.69–1.82 (m, 5H), 1.97–2.04 (m, 0.5H), 2.05–2.14 (m, 1H), 2.37 (t, J = 8.2 Hz, 0.5H), 2.59–2.73 (m, 2H), 2.75–2.92 (m, 4H), 2.97 (dd, J = 7.1 Hz, J = 13.7 Hz, 1H), 3.06 (dd, J = 7.2 Hz, J = 13.6 Hz, 1H), 3.23–3.35 (m, 5H), 3.35–3.40 (m, 1H), 4.55 (dd, J = 7.1 Hz, J = 14.4 Hz, 1H), 6.36 (d, J = 6.0 Hz, 1H), 6.77 (d, J = 8.3 Hz, 1H), 6.83–6.90 (m, 2H), 6.96 (t, J = 8.5 Hz, 2H), 7.11–7.22 (m, 3H), 7.24–7.26 (m, 1H). 13C-NMR (CDCl3): δ 17.7, 24.4, 25.7, 29.2, 29.7, 30.7, 37.7, 45.1, 47.7, 49.5, 52.4, 54.6, 55.6, 114.4, 115.3, 116.5, 120.5, 130.3, 130.9, 132.6, 135.1, 151.3, 160.9, 162.8, 171.4, 176.4. HRMS calcd. for (M + H)+: C29H39ClFN4O2 529.2746, found 529.2741.
2-[(Benzoyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}octanamide (3y): Product 3y was obtained from resin 23, 1-iodohexane, benzoic acid, and 3-chlorophenylpiperazine as brown oil (10.3 mg, 41% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (94:6). Initial LC/MS purity 68%, tR = 11.5 min; 1H-NMR (CDCl3): δ 0.78–0.89 (m, 3H), 1.10–1.41 (m, 9H), 1.68–1.81 (m, 1H), 1.84–1.94 (m, 2H), 1.97–2.08 (m, 0.4H), 2.37 (t, J = 8.1 Hz, 0.4H), 2.70–2.80 (m, 2H), 2.81–2.93 (m, 3H), 3.23–3.46 (m, 6H), 4.38–4.69 (m, 1H), 6.76 (d, J = 8.6 Hz, 1H), 6.86 (d, J = 8.4 Hz, 2H), 7.05 (brs, 1H), 7.17 (t, J = 8.0 Hz, 1H), 7.42 (t, J = 7.5 Hz, 2H), 7.49 (t, J = 7.3 Hz, 1H), 7.59 (brs, 1H), 7.83 (d, J = 7.4 Hz, 2H). 13C-NMR (CDCl3): δ 14.0, 17.7, 22.5,, 29.0, 31.6, 32.7, 37.8, 47.7, 52.5, 54.2, 55.9, 114.4, 116.4, 120.4, 127.2, 128.6, 130.2, 131.8, 133.7, 135.1, 151.4, 167.4, 172.4. HRMS calcd. for (M + H)+: C28H40ClN4O2 499.2840, found 499.2840.
2-[(Benzoyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}-3(4-methoxyphenyl)-propanamide (3z): Product 3z was obtained from resin 23, 4-methoxybenzyl chloride, benzoic acid, and 3-chlorophenyl piperazine as an amorphous white solid (5.2 mg, 19% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (94:6). Initial LC/MS purity 59%, tR = 9.4 min; 1H -MR (CDCl3): δ 1.64–1.75 (m, 2H), 2.43–2.73 (m, 6H), 3.05–3.21 (m, 6H), 3.26–3.39 (m, 2H), 3.78 (s, 3H), 4.69 (dd, J = 7.5 Hz, J = 13.7 Hz, 1H), 6.74 (d, J = 7.4 Hz, 1H), 6.79–6.87 (m, 4H), 6.92 (d, J = 6.0 Hz, 1H), 7.08 (brs, 1H), 7.14–7.21 (m, 3H), 7.39 (t, J = 7.6 Hz, 2H), 7.48 (t, J = 7.4 Hz, 1H), 7.74 (d, J = 7.3 Hz, 2H). 13C-NMR (CDCl3): δ 24.6, 37.9, 38.5, 48.2, 52.7, 55.3, 55.6, 56.6, 114.1, 116.1, 119.9, 127.1, 128.6, 128.7, 130.1, 130.4, 131.8, 133.7, 135.0, 151.7, 158.7, 167.0, 170.9. HRMS calcd. for (M + H)+: C30H36ClN4O3 535.2476, found 535.2470.
2-[(Benzoyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}-3-(3-fluorophenyl)-propanamide (3aa): Product 3aa was obtained from resin 23, 3-fluorobenzyl chloride, benzoic acid, and 3-chlorophenylpiperazine as light yellow oil (9.2 mg, 35% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (94:6). Initial LC/MS purity 61%, tR = 9.9 min; 1H- NMR (CDCl3): δ 1.61–1.77 (m, 2H), 1.96–2.07 (m, 1H), 2.37 (t, J = 8.1 Hz, 1H), 2.49–2.54 (m, 1H), 2.55–2.63 (m, 2H), 2.64–2.69 (m, 1H), 2.84 (s, 1H), 3.14–3.19 (m, 4H), 3.25–3.40 (m, 3H), 4.64–4.85 (m, 1H), 6.75 (d, J = 7.6 Hz, 1H), 6.85 (d, J = 7.4 Hz, 2H), 6.91–7.01 (m, 2H), 7.02–7.09 (m, 2H)), 7.14–7.21 (m, 1H), 7.22–7.26 (m, 1H), 7.29–7.36 (m, 1H), 7.39 (t, J = 7.6 Hz, 2H), 7.46–7.52 (m, 1H), 7.75 (d, J = 7.8 Hz, 2H). 13C-NMR (CDCl3): δ 17.7, 14.5, 19.6, 30.7, 38.4, 48.1, 49.5, 52.6, 55.3, 56.4, 113.9, 114.0, 114.2, 116.2, 116.4, 119.9, 125.1, 127.1, 128.6, 130.1, 131.9, 133.5, 135.0, 139.4, 151.7, 161.9, 163.8, 167.2, 170.8. HRMS calcd. for (M + H)+: C29H33ClFN4O2 523.2276, found 523.2283.
2-[(Benzoyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}-3-(4-fluorophenyl)-propanamide (3ab): Product 3ab was obtained from resin 23, 4-fluorobenzyl bromide, benzoic acid, and 3-chloro-phenylpiperazine as an amorphous light yellow solid (14.2 mg, 54% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (94:6). Initial LC/MS purity 73%, tR = 9.8 min; 1H-NMR (CD3OD/CDCl3): δ 1.74–1.85 (m, 2H), 1.97–2.07 (m, 1H), 2.37 (t, J = 8.1 Hz, 1H), 2.63–2.76 (m, 2H), 2.82–2.92 (m, 3H), 3.09 (dd, J = 7.2 Hz, J = 13.5 Hz, 1H), 3.16 (dd, J = 7.4 Hz, J = 13.7 Hz, 1H), 3.23–3.34 (m, 5H), 4.63–4.84 (m, 1H), 6.72–6.81 (m, 1H), 6.82–6.92 (m, 2H), 6.97 (t, J = 8.6 Hz, 2H), 7.09–7.26 (m, 3H), 7.41 (t, J = 7.6 Hz, 2H), 7.47–7.53 (m, 1H), 7.76 (d, J = 7.8 Hz, 2H). 13C-NMR (CD3OD/CDCl3): δ 17.6, 24.3, 29.6, 30.7, 37.5, 47.5, 52.3, 55.4, 114.5, 115.4, 115.5, 116.6, 120.6, 127.2, 128.6, 130.3, 130.8, 130.9, 132.0, 132.4, 133.3, 135.1, 151.2, 160.9, 162.9, 167.6, 168.8. HRMS calcd. for (M + H)+: C29H33ClFN4O2 523.2276, found 523.2252.
2-[(Quinolin-2-oyl)amino]-N-{[4-(3-chloroyphenyl)-piperazin-1-yl]propyl}octanamide (3ac): Product 3ac was obtained from resin 23, 1-iodohexane, quinaldic acid, and 3-chlorophenylpiperazine as brown oil (12.4 mg, 45% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (95:5). Initial LC/MS purity 72%, tR = 13.3 min; 1H-NMR (CDCl3): δ 0.86 (t, J = 6.9 Hz, 3H), 1.24–1.32 (m, 5H), 1.34–1.51 (m, 4H), 1.79–1.93 (m, 1H), 1.96–2.09 (m, 3H), 2.84 (s, 1H), 2.92–3.19 (m, 4H), 3.33–3.48 (m, 6H), 4.53 (dd, J = 7.6 Hz, 13.7 Hz, 1H), 6.72 (dd, J = 2.1 Hz, J = 8.3 Hz, 1H), 6.78–6.84 (m, 1H), 6.89 (d, J = 7.9 Hz, 1H), 7.17 (t, J = 8.1 Hz, 1H), 7.39 (brs, 1H), 7.58–7.66 (m, 1H), 7.72–7.81 (m, 1H), 7.86 (d, J = 8.1 Hz, 1H), 8.14 (d, J = 8.4 Hz, 1H), 8.20 (d, J = 8.5 Hz, 1H), 8.28 (d, J = 8.5 Hz, 1H), 8.63 (d, J = 7.5 Hz, 1H). 13C-NMR (CDCl3): δ 14.0, 17.7, 22.5, 25.9, 28.9, 31.6, 32.1, 36.9, 46.9, 52.1, 54.3, 55.2, 114.6, 116.7, 118.6, 120.9, 127.7, 128.2, 129.4, 129.9, 130.3, 135.1, 137.6, 146.5, 148.9, 150.9, 164.9, 172.4. HRMS calcd. for (M + H)+: C31H41ClN5O2 550.2949, found 550.2936.
2-[(Quinolin-2-oyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}-3-(4-methoxyphenyl)-propanamide (3ad): Product 3ad was obtained from resin 23, 4-methoxybenzyl chloride, quinaldic acid, and 3-chlorophenylpiperazine as brown oil (8.0 mg, 27% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (94:6). Initial LC/MS purity 58%, tR = 11.3 min; 1H-NMR (CDCl3): δ 1.62–1.73 (m, 2H), 1.97–2.06 (m, 1H), 2.37 (t, J = 8.2 Hz, 1H), 2.42–2.47 (m, 1H), 2.48–2.59 (m, 3H), 2.84 (s, 1H), 2.97–3.07 (m, 3H), 3.17 (dd, J = 7.6 Hz, J = 13.7 Hz, 1H), 3.26 (dd, J = 5.8 Hz, J = 13.8 Hz, 1H), 3.32–3.39 (m, 2H), 3.78 (s, 3H), 4.76 (dd, J = 7.5 Hz, J = 14.0 Hz, 1H), 6.62 (d, J = 8.3 Hz, 1H), 6.69 (brs, 1H), 6.79 (d, J = 7.4 Hz, 1H), 6.86 (d, J = 8.4 Hz, 2H), 7.04–7.14 (m, 2H), 7.22–7.26 (m, 2H), 7.61 (t, J = 7.5 Hz, 1H), 7.69–7.77 (m, 1H), 7.84 (d, J = 8.2 Hz, 1H), 8.05 (d, J = 8.5 Hz, 1H), 8.16–8.28 (m, 2H), 8.75 (d, J = 7.9 Hz, 1H). 13C-NMR (CDCl3): δ 17.7, 24.7, 29.6, 30.7, 37.5, 47.9, 49.5, 52.8, 55.4, 113.8, 114.1, 115.7, 118.7, 127.7, 128.2, 128.8, 129.4, 129.8, 130.0, 130.2, 130.6, 134.9, 137.6, 146.4, 148.9, 156.6, 158.7, 164.6, 170.7. HRMS calcd. for (M + H)+: C33H37ClN5O3 586.2585, found 586.2600.
2-[(Quinolin-2-oyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}-3-(3-fluorophenyl)-propanamide (3ae): Product 3ae was obtained from resin 23, 3-fluorobenzyl chloride, quinaldic acid, and 3-chlorophenylpiperazine as brown oil (9.4 mg, 33% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (95:5). Initial LC/MS purity 59%, tR = 11.6 min; 1H- NMR (CDCl3): δ 1.58–1.70 (m, 2H), 1.95–2.08 (m, 1H), 2.35–2.42 (m, 2H), 2.43–2.50 (m, 3H), 2.84 (s, 1H), 2.94–3.03 (m, 3H), 3.21–3.27 (m, 1H), 3.29–3.40 (m, 3H), 4.81 (dd, J = 7.9 Hz, J = 14.0 Hz, 1H), 6.92–7.00 (m, 1H), 7.04–7.13 (m, 3H), 7.17–7.22 (m, 1H), 7.27–7.32 (m, 1H), 7.57–7.64 (m, 1H), 7.71–7.77 (m, 1H), 7.84 (d, J = 8.2 Hz, 1H), 8.06 (d, J = 8.5 Hz, 1H), 8.15–8.28 (m, 2H), 8.73 (d, J = 8.4 Hz, 1H). 13C-NMR (CDCl3): δ 17.7, 24.7, 29.6, 30.7, 38.1, 39.2, 48.1, 52.9, 54.9, 57.0, 113.6, 113.9, 114.0, 115.5, 116.4, 116.6, 118.6, 119.3, 125.4, 127.7, 128.2, 129.4, 129.8, 129.9, 130.3, 134.9, 137.6, 139.4, 146.4, 148.8, 151.7, 161.9, 163.9, 164.6, 170.2. HRMS calcd. for (M + H)+: C32H34ClFN5O2 574.2385, found 574.2402.
2-[(Quinolin-2-oyl)amino]-N-{[4-(3-chlorophenyl)-piperazin-1-yl]propyl}-3-(4-fluorophenyl)-propanamide (3af). Product 3af was obtained from resin 23, 4-fluorobenzyl bromide, quinaldic acid, and 3-chlorophenylpiperazine as brown oil (15.5 mg, 54% isolated yield) following chromatographic purification over silica gel with CH2Cl2–MeOH (93:7). Initial LC/MS purity 72%, tR = 11.5 min; 1H- NMR (CDCl3): δ 1.84–1.98 (m, 2H), 1.98–2.05 (m, 0.5H), 2.37 (t, J = 8.1 Hz, 0.5H), 2.79–3.17 (m, 5H), 3.20–3.44 (m, 8H), 4.79 (dd, J = 7.2 Hz, J = 14.7 Hz, 1H), 6.71 (dd, J = 2.1 Hz, J = 8.3 Hz, 1H), 6.78–6.84 (m, 1H), 6.89 (dd, J = 1.1 Hz, J = 7.9 Hz, 1H), 6.96–7.04 (m, 2H), 7.17 (t, J = 8.1 Hz, 1H), 7.27–7.40 (m, 3H), 7.57–7.66 (m, 1H), 7.72–7.81 (m, 1H), 7.85 (d, J = 8.1 Hz, 1H), 8.07 (d, J = 8.5 Hz, 1H), 8.16 (d, J = 8.5 Hz, 1H), 8.26 (d, J = 8.5 Hz, 1H), 8.72 (d, J = 7.8 Hz, 1H). 13C-NMR (CDCl3): δ 17.7, 23.9, 29.6, 30.7, 37.1, 37.4, 46.9, 49.4, 52.0, 55.2, 114.5, 115.4, 115.6, 116.7, 118.5, 120.9, 127.7, 128.2, 129.4, 129.8, 130.3, 131.0, 132.4, 135.1, 137.6, 146.5, 148.8, 150.8, 160.9, 162.9, 164.8, 171.2. HRMS calcd. for (M + H)+: C32H34ClFN5O2 574.2385, found 574.2376.