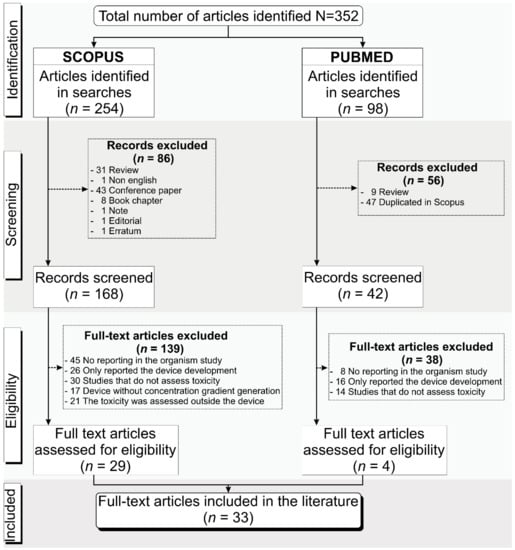
1
Institute of Chemical Biology and Fundamental Medicine, Siberian Branch, Russian Academy of Sciences, Lavrentyev Avenue 8, Novosibirsk 630090, Russia
2
Novosibirsk Research Institute of Traumatology and Orthopedics n.a. Ya.L. Tsivyan, Department of Neurosurgery, Frunze Street 17, Novosibirsk 630091, Russia
Cells 2022, 11(19), 3106; https://doi.org/10.3390/cells11193106 - 2 Oct 2022
Cited by 9 | Viewed by 3003
Abstract
Glioma is the most common and heterogeneous primary brain tumor. The development of a new relevant preclinical models is necessary. As research moves from cultures of adherent gliomas to a more relevant model, neurospheres, it is necessary to understand the changes that cells
[...] Read more.
Glioma is the most common and heterogeneous primary brain tumor. The development of a new relevant preclinical models is necessary. As research moves from cultures of adherent gliomas to a more relevant model, neurospheres, it is necessary to understand the changes that cells undergo at the transcriptome level. In the present work, we used three patient-derived gliomas and two immortalized glioblastomas, while their cultivation was carried out under adherent culture and neurosphere (NS) conditions. When comparing the transcriptomes of monolayer (ML) and NS cell cultures, we used Enrichr genes sets enrichment analysis to describe transcription factors (TFs) and the pathways involved in the formation of glioma NS. It was observed that NS formation is accompanied by the activation of five common gliomas of TFs, SOX2, UBTF, NFE2L2, TCF3 and STAT3. The sets of transcripts controlled by TFs MYC and MAX were suppressed in NS. Upregulated genes are involved in the processes of the epithelial–mesenchymal transition, cancer stemness, invasion and migration of glioma cells. However, MYC/MAX-dependent downregulated genes are involved in translation, focal adhesion and apical junction. Furthermore, we found three EGFR and FGFR signaling feedback regulators common to all analyzed gliomas—SPRY4, ERRFI1, and RAB31—which can be used for creating new therapeutic strategies of suppressing the invasion and progression of gliomas.
Full article
(This article belongs to the Section Cells of the Nervous System)
▼
Show Figures