Catalysts 2022, 12(12), 1512; https://doi.org/10.3390/catal12121512 - 25 Nov 2022
Cited by 6 | Viewed by 1896
Abstract
The homogeneous photocatalytic degradation of model pesticide clopyralid (CLPR) has been investigated under various experimental setups. Lab-scale experiments under UV-A radiation in an acidic environment showed that the degradation rate generally increased when increasing either Fe3+ or H2O2 concentration
[...] Read more.
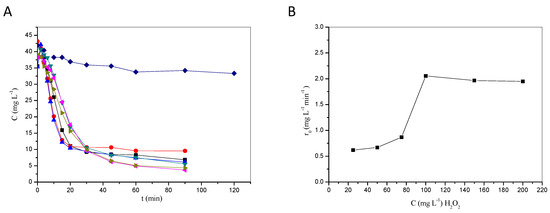
The homogeneous photocatalytic degradation of model pesticide clopyralid (CLPR) has been investigated under various experimental setups. Lab-scale experiments under UV-A radiation in an acidic environment showed that the degradation rate generally increased when increasing either Fe3+ or H2O2 concentration up to a point beyond which (i.e., 100 mg L−1 for peroxide or 7 mg L−1 for ferric ions) Fenton reagents had little or even detrimental effect on degradation. Thus, there is an optimum concentration of Fenton reagents for maximizing treatment performance, beyond which degradation rates are not enhanced. Excessive concentrations of peroxide and/or catalyst may (i) introduce unnecessary treatment costs, (ii) reduce performance due to scavenging effects, and (iii) raise environmental concerns associated with the disposal of, e.g., high concentrations of iron in the receiving water courses. Switching from UV-A to visible light led to similar rates of degradation, i.e., 86% and 82.2%, respectively, after 90 min of reaction, highlighting the potential of using renewable energy, i.e., natural sunlight, to drive the process. Treatment for 120 min also led to 90% mineralization and quantitative release of nitrogen originally present in the pesticide; this was also accompanied by complete elimination of eco-toxicity to Vibrio fischeri. Pilot-scale experiments were performed in a fountain-type reactor using a commercial pesticide formulation containing CLPR. Both the degradation and mineralization rates increased with increasing the intensity of the incident UV-A radiation from 1.88 to 4.03 mW cm−2. Experiments were also conducted with different liquid volumes, i.e., from 3 to 8 L. Illumination of 5 L wastewater resulted in 80% mineralization after 60 min and this only slightly decreased to 73% at 8 L. Overall, the findings underline the promising perspectives of the application of the treatment method in upgrading the quality of water and liquid waste containing pesticides.
Full article
(This article belongs to the Special Issue Applications of Heterogeneous Catalysts in Green Chemistry)
►
Show Figures