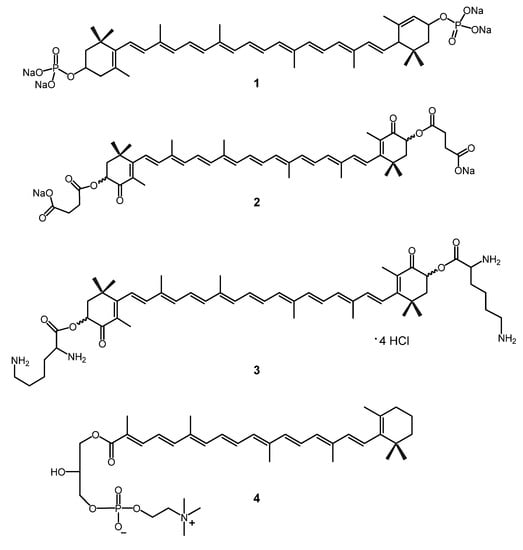
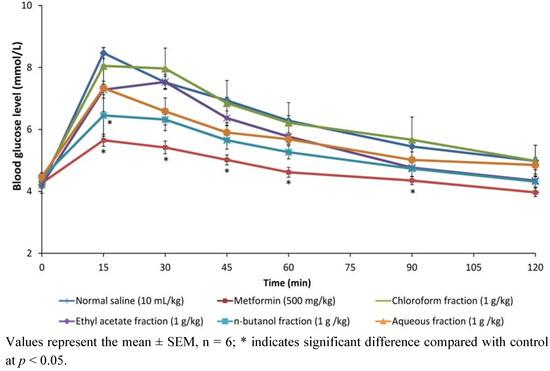
Molecules 2012, 17(5), 5164-5176; https://doi.org/10.3390/molecules17055164 - 4 May 2012
Cited by 36 | Viewed by 7069
Abstract
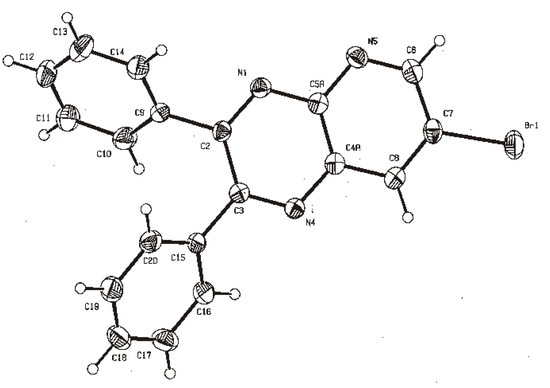
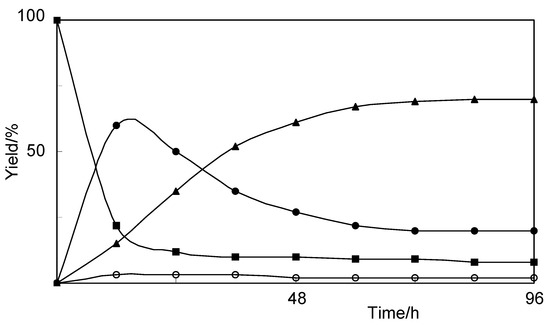
We report herein the microwave assisted synthesis, without solvents and catalysts, of 6-substituted quinoxalines and 7-substituted pyrido[2,3b]pyrazines. The compounds were obtained in good yields and short reaction times using the mentioned procedure and two new structures are reported. A complete 1
[...] Read more.
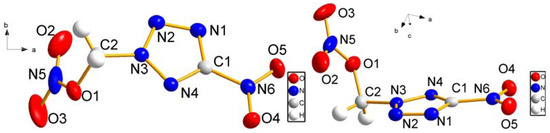
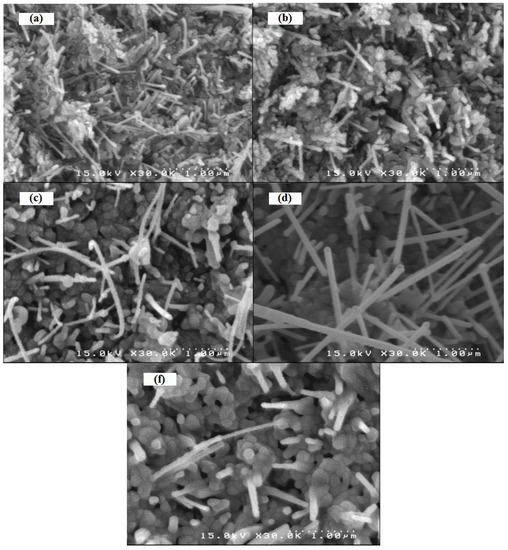
We report herein the microwave assisted synthesis, without solvents and catalysts, of 6-substituted quinoxalines and 7-substituted pyrido[2,3b]pyrazines. The compounds were obtained in good yields and short reaction times using the mentioned procedure and two new structures are reported. A complete 1H- and 13C-NMR assignment was performed using 1D and 2D-NMR. Additionally, an in vitro screening was performed on Gram-positive and Gram-negative bacteria using amoxicillin as positive reference. Compounds bearing a pyridyl group tended to have higher antibacterial activity, but the best activity against Bacillus subtilis and Proteus mirabilis was observed with quinoxaline derivatives.
Full article
(This article belongs to the Section Medicinal Chemistry)
►
Show Figures