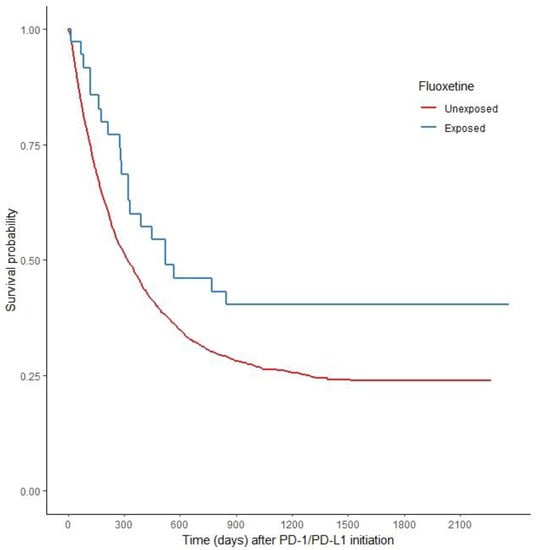
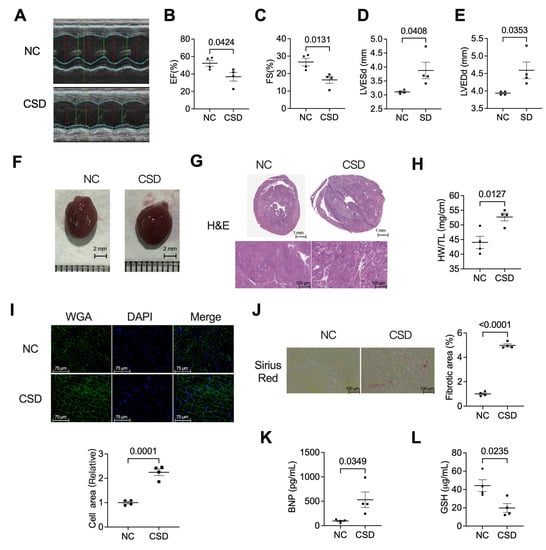
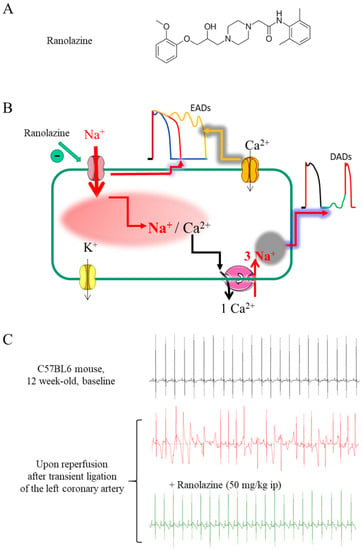
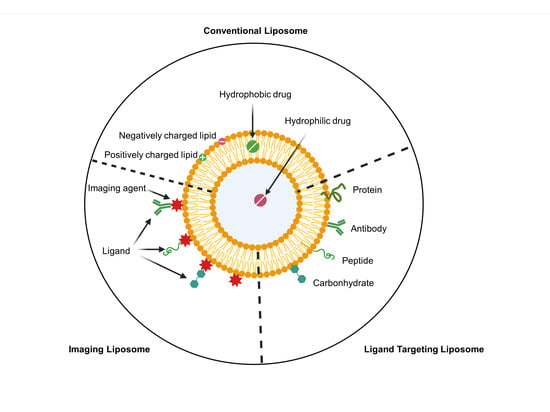
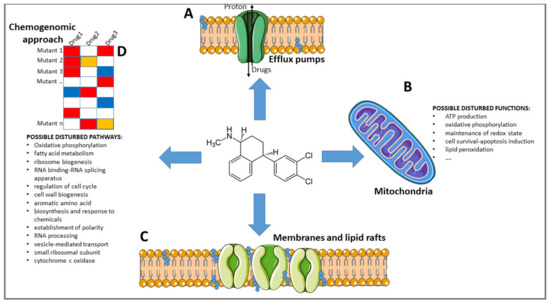
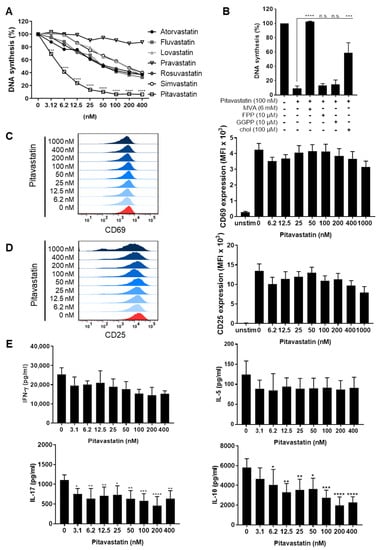
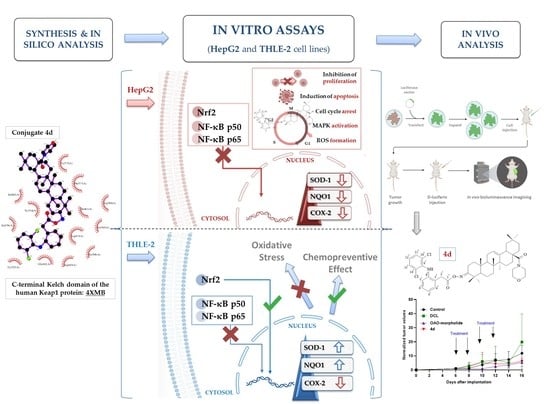
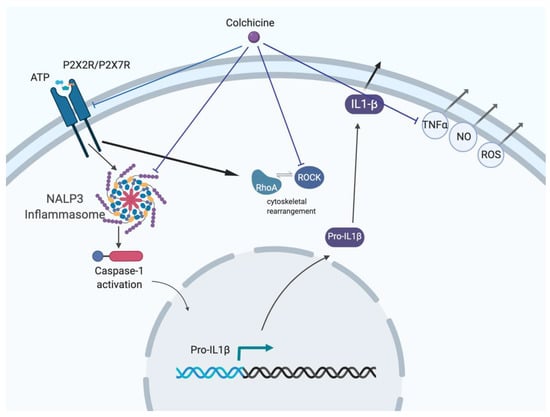
Old Pharmaceuticals with New Applications (Closed)
A topical collection in Pharmaceuticals (ISSN 1424-8247). This collection belongs to the section "Pharmacology".
Viewed by 175286Editor
Interests: drug discovery; Eph; ephrin; protein–protein interaction inhibitors (PPI-is); polyphenols; cancer; platelets
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The decline or leveling out of output of the R&D programs from pharmaceutical companies may have begun to turn a corner compared to the early years of the 21st century. Although one reason for this increase is immunopharmacology-based treatments, small molecules still play an important role. Medicinal chemistry approaches to find small molecule lead compounds with the desired pharmacological activity continue to use natural products, synthesis, and existing drugs as sources.
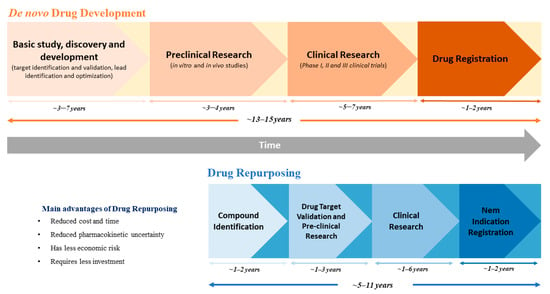
In recent years, we have experienced a surge of interest in drug repositioning. There is a trend in finding new uses for existing drugs, especially in diseases where there is an unmet clinical need such as neglected and orphan diseases. Another opportunity is developing novel applications using a combination of old drugs.
The most fruitful basis for the discovery of a new drug is to start with an old drug” goes a famous statement from Sir James Black, which has received many adherents this century, not only in finding new applications but also looking for the unexploited potential of old drugs as starting points for molecular modifications.
The journal Pharmaceuticals invites both reviews and original articles shedding light on the challenges and opportunities of using old pharmaceuticals in drug discovery. Topics include: drug repositioning, selective optimization of side effects, drug metabolites as sources of new drugs, old drug combinations, beyond pharmaceuticals applications. The collection of manuscripts will be published as a Special Issue of the journal.
Dr. Massimiliano Tognolini
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pharmaceuticals is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- drug repositioning
- drug discovery
- old drugs
- sosa
- drug metabolism
- drug combinations
Related Special Issue
- Drug Repositioning in Pharmaceuticals (2 articles)