Sol-Gel Technique
A special issue of Materials (ISSN 1996-1944). This special issue belongs to the section "Manufacturing Processes and Systems".
Deadline for manuscript submissions: closed (15 December 2010) | Viewed by 65382
Special Issue Editor
Interests: reaction engineering of organic vapor phase deposition; dynamics of polymer electrolyte membrane fuel cells; recovery of thiols for hydrocarbon streams; developing a large-scale liquid scintillation detector
Special Issues, Collections and Topics in MDPI journals
Special Issue Information
Dear Colleagues,
Sol-gels are versatile materials made by condensing a solution (sol) of metal oxide precursors into three dimensional networks. The gels are bi-phasic systems in which a continuous fluid phase fills the space inside a polymerized network. The gels can be dried in controlled fashion to produce porous solids with unique thermal, mechanical, optical and chemical properties. Sol-gel materials have grown in importance over the past 30 years as chemists and engineers have learned how to vary the reactants and processing conditions to tailor material properties for specific applications.
The early work with sol-gels focused on those made of silica, derived by condensation of silanols groups (Si-OH), as illustrated in by reaction (1).
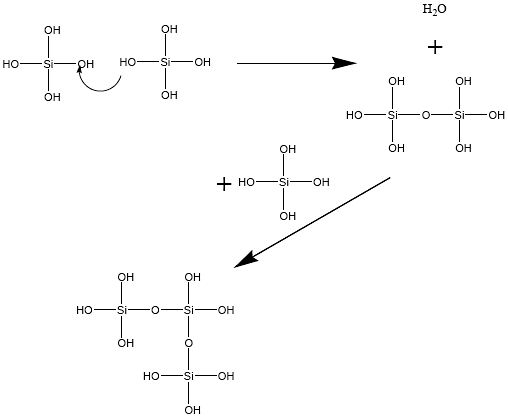
Reaction 1. Condensation of silanols into a gel. The silanols condense by forming water leading to a network of Si-O-Si bonds. The quaternary functionality of the Si results in a three dimensional network.
The silanols groups may be on the surface of nanometer sized silica particles or could be formed by hydrolysis of silicone alkoxides as illustrated in reaction (2).
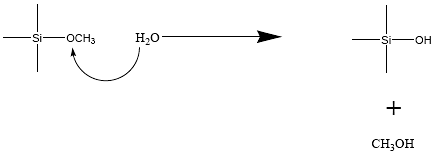
Reaction 2. Hydrolysis of silicon alkoxides to produce silanols. The silanols subsequently undergo condensation reactions to produce silica gels.
Silicon has four functional groups that can undergo condensation. By altering the reaction conditions (temperature, concentration, pH, solvent and reactant), the degree and nature of the condensation reactions can be controlled. At low pH conditions the rate of condensation slows down with degree of branching resulting in low cross-link density and very porous gels. In contrast high pH will accompany rapid condensation that can produce dense particles that precipitate from the solution. The gels are filled with liquid, generally a water alcohol solution. Controlled drying of the gel is employed to tailor the porosity and composition for specific applications. Highly porous materials can be produced that are exceptional thermal insulators. Alternatively dense gels may be employed as thin film protective coatings for lenses.
Advances in chemistry and the chemical precursors available for sol-gel processing have made it an extremely flexible process for materials synthesis. We are no longer limited to silica gels or even to metals oxides. It is now possible to make sol gels materials from almost any transition metal, as well as make composite materials. The applications of sol-gel materials have grown as the synthesis and processing methods have opened new vistas of material properties. A list of some of major applications of sol-gels is given in Table 1.
Table 1. Applications of Sol-gel materials.
|
Application
|
Sol-Gel Material
|
|
Optical fibers
|
High purity doped silica gel films for optical fiber precursors
|
|
Protective optical coatings
|
Abrasion resistance silica gel coatings on plastic substrates
|
|
Anti-reflective optical coatings
|
Laser windows, smart windows
|
|
Thermal insulation for windows
|
Aerogel window spacers, solar collector coatings
|
|
High Temperature Refractory Insulation
|
Ceramic foams
|
|
Chemical Sensors
|
Thin film NOx sensors, sol-gel coated crystal oscillators
|
|
Catalysts and Adsorbents
|
Silica alumina solid acid catalysts, high surface area catalyst supports, Silica gel desiccant
|
|
Ceramic membranes
|
Sol-gel molecular sieves, antibacterial filters
|
|
Abrasives
|
Alumina abrasives
|
|
Dental sealants and fillers
|
Hydroxyapatite
|
Sol-gel technology offers many important advantages in materials processing. The nanometer structure of the gels permits low temperature processing of ceramic materials so that ceramics and plastics can be combined in hybrid materials. The introduction of metal alkoxides precursors for sol-gels made possible the production of high purity materials that dramatically improved the quality of optical fibers. The pore structure and large surface areas associated with sol-gel materials has been essential to the development of catalysts and adsorbents making possible improved production of gasoline and removing impurities for automobile exhausts and new photocatalysts for splitting water. We now have a tool box of chemical and processing methods to tailor sol-gels to tackle new materials technologies. In this special issue we present reviews of the synthesis and processing techniques to produce sol-gel materials.
Prof. Dr. Jay B. BenzigerGuest Editor
Keywords
- silica gels
- aerogels
- xerogels
- optical fiber cladding
- sol gel derived waveguides
- abrasive resistant coatings
- hybrid glass coatings
- antireflective coatings
- titania sol gel photocatalysts
- aerogel windows
- sol gel catalysts
- adsorbents
- ceramic foams
- sol gel membranes
- sol gel NOx sensors
- colloidal gels
- sol gel transition
- self cleaning coatings
- ceramic spin coating
- ceramic dip coating
- sol gel composites
Benefits of Publishing in a Special Issue
- Ease of navigation: Grouping papers by topic helps scholars navigate broad scope journals more efficiently.
- Greater discoverability: Special Issues support the reach and impact of scientific research. Articles in Special Issues are more discoverable and cited more frequently.
- Expansion of research network: Special Issues facilitate connections among authors, fostering scientific collaborations.
- External promotion: Articles in Special Issues are often promoted through the journal's social media, increasing their visibility.
- Reprint: MDPI Books provides the opportunity to republish successful Special Issues in book format, both online and in print.
Further information on MDPI's Special Issue policies can be found here.
Related Special Issue
- Sol-Gel Technique in International Journal of Molecular Sciences (5 articles)






