Sol-Gel Synthesis of Non-Silica Monolithic Materials
Abstract
:1. Introduction
2. Synthesis of Monolithic Materials
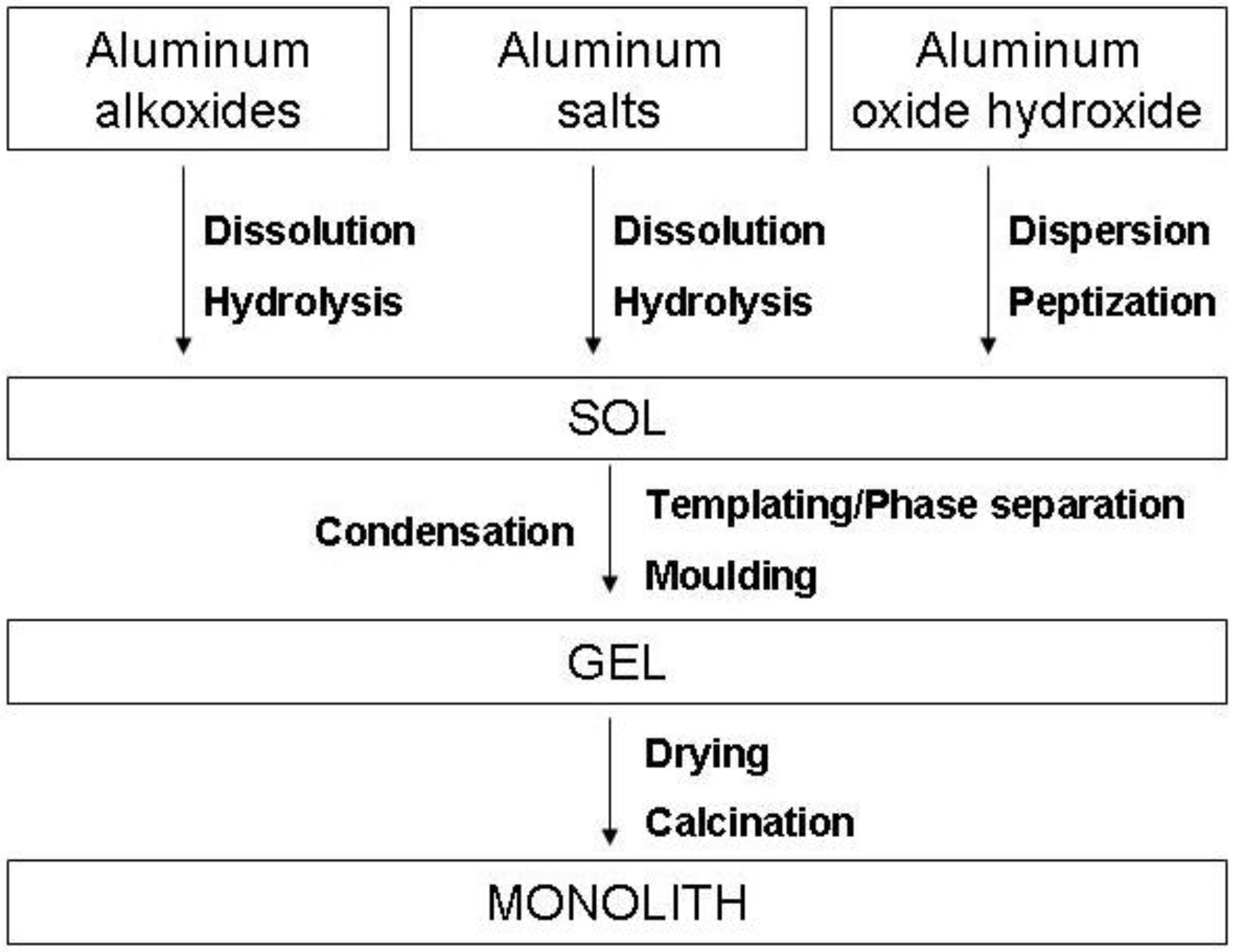
2.1. The Sol-gel method
- a)
- hydrolysis of precursors-sol formation
- b)
- polycondensation of hydrolyzed precursors-gelation
- c)
- aging
- d)
- drying
- e)
- calcination



2.2. Phase separation. Template-free synthesis

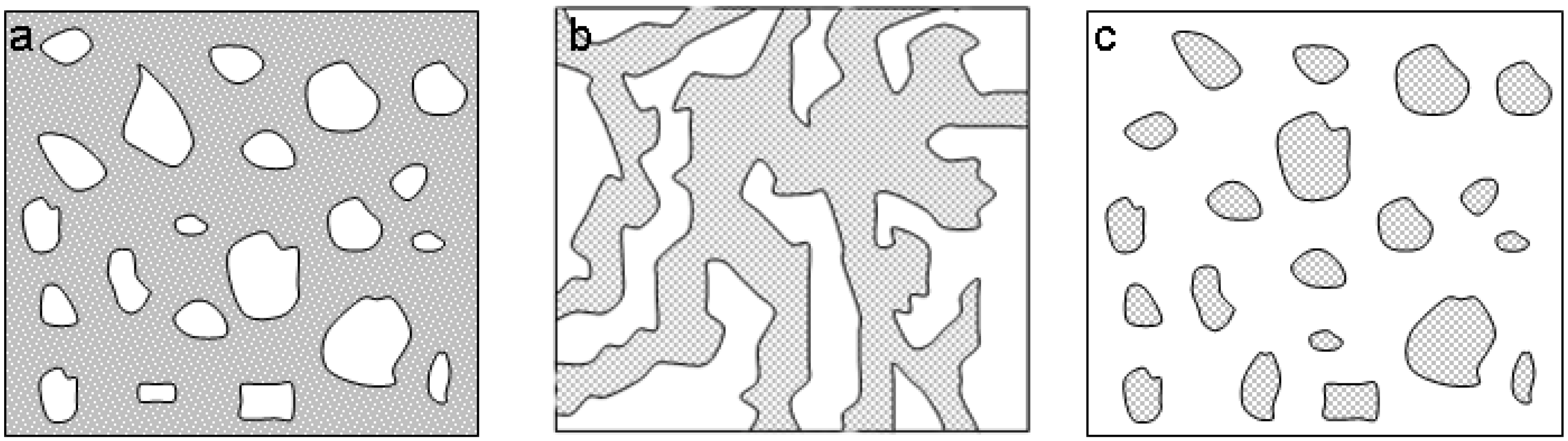
2.3. Synthesis with template
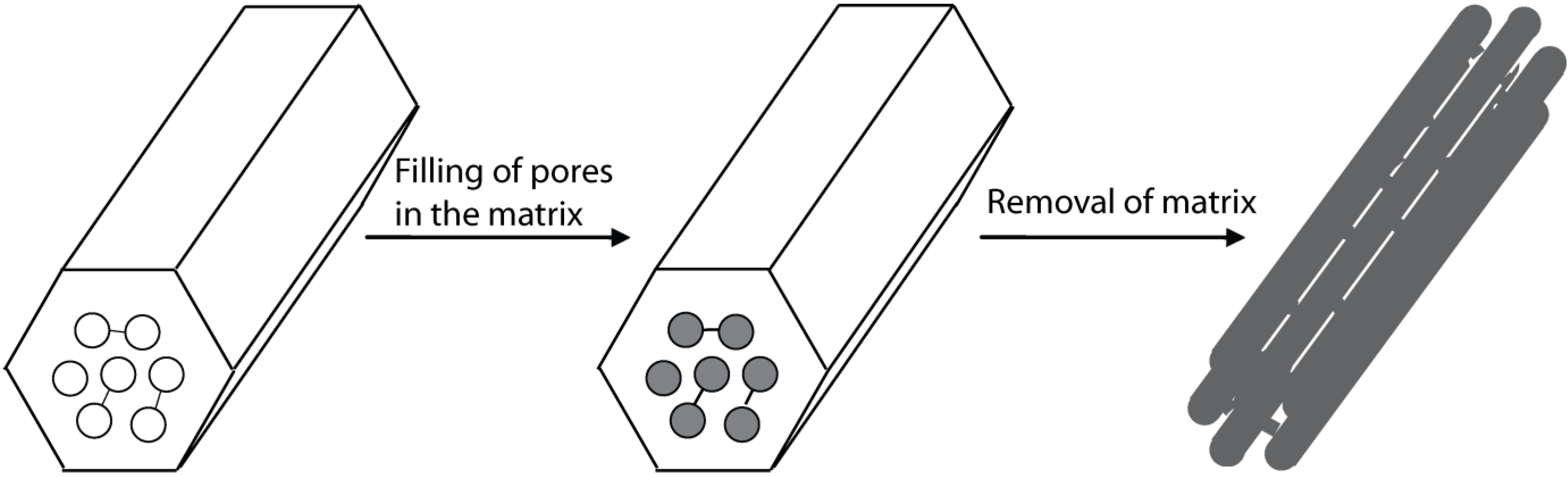
2.4. The Replica method
3. Monolithic Oxides
3.1. Alumina
3.2. Zirconia
3.3. Titania
3.4. Other materials
| Material | Preparation Method |
|---|---|
| LiFePO4/Carbon Composite [11] | replica |
| CdSe/ZnS [12] | direct gelation |
| Ag2Se [13] | direct gelation |
| CdS-Ag [103] | direct gelation |
| MnO2, Mn2O3, SnO2, Co3O4 [42,104] | replica |
| MgO [105] | direct gelation |
| Fe2O3 [106] | direct gelation |
| FeOOH [107] | direct gelation |
| NiO-ZnO [108] | direct gelation |
| SnO2 [20] | direct gelation |
| ZnO [109] | direct gelation |
| Silver [110] | template synthesis |
| Carbon [40,111,112,113] | replica |
| SiC [114] | replica |
| MgAl2O4 [115] | replica |
4. Conclusions
Acknowledgements
References and Notes
- Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials. IUPAC Recommendations 2007, 1812.
- Siouffi, A.-M. Silica gel-based monoliths prepared by the sol-gel method: facts and figures. J. Chromatogr. A 2003, 801–818. [Google Scholar] [CrossRef]
- Hu, J.W.; Li, X.G.; Cai, Y.W.; Han, H.Y. Hybrid silica polymeric monolith-based in-tube microextraction and CE for determination of bisphenol A in beverages. J. Sep. Sci. 2009, 32, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Sakai-Kato, K.; Toyo'oka, T. Silica sol-gel monolithic materials and their use in a variety of applications. J. Sep. Sci. 2005, 28, 1893–1908. [Google Scholar] [CrossRef]
- Kim, Y.S.; Guo, X.F.; Kim, G.J. Asymmetric Ring Opening Reaction of Catalyst Immobilized on Silica Monolith with Bimodal Meso/Macroscopic Pore Structure. Top. Catal. 2009, 52, 197–204. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tanaka, N. Sol-gel with phase separation. Hierarchically porous materials optimized for high-performance liquid chromatography separations. Acc. Chem. Res. 2007, 40, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Randon, J.; Guerrin, J.F.; Rocca, J.L. Synthesis of titania monoliths for chromatographic separations. J. Chromatogr. A 2008, 1214, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Randon, J.; Huguet, S.; Piram, A.; Puy, G.; Demesmay, C.; Rocca, J.L. Synthesis of zirconia monoliths for chromatographic separations. J. Chromatogr. A 2006, 1109, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, C.Y.; Liang, H.; Liu, Y. Macroporous Monolithic Pt/gamma-Al2O3 and K-Pt/gamma-Al2O3 Catalysts Used for Preferential Oxidation of CO. Catal. Lett. 2009, 127, 339–347. [Google Scholar] [CrossRef]
- Fan, L.Z.; Hu, Y.S.; Maier, J.; Adelhelm, P.; Smarsly, B.; Antonietti, M. High electroactivity of polyaniline in supercapacitors by using a hierarchically porous carbon monolith as a support. Adv. Funct. Mater. 2007, 17, 3083–3087. [Google Scholar] [CrossRef]
- Doherty, C.M.; Caruso, R.A.; Smarsly, B.M.; Adelhelm, P.; Drummond, C.J. Hierarchically Porous Monolithic LiFePO4/Carbon Composite Electrode Materials for High Power Lithium Ion Batteries. Chem. Mater. 2009, 21, 5300–5306. [Google Scholar] [CrossRef]
- Arachchige, I.U.; Brock, S.L. Highly luminescent quantum-dot monoliths. J. Am. Chem. Soc. 2007, 129, 1840–1841. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.H.; Arachchige, I.U.; Brock, S.L. Expanding the Repertoire of Chalcogenide Nanocrystal Networks: Ag2Se Gels and Aerogels by Cation Exchange Reactions. J. Am. Chem. Soc. 2009, 131, 2800–2801. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Jimenez-Sandoval, S.; Estevez, M.; Pacheco, S.; Vargas, S. Photo-quenched luminescence in Co(II)-doped sol-gel zirconia. J. Sol-Gel Sci. Technol. 2007, 44, 97–104. [Google Scholar] [CrossRef]
- Tomás, S.A.; Zelaya, O.; Palomino, R.; Lozada, R.; García, O.; Yáñez, J.M.; Ferreira da Silva, A. Optical characterization of sol gel TiO2 monoliths doped with Brilliant Green. Eur Phys J Spec Top 2008, 153, 255–258. [Google Scholar] [CrossRef]
- El Mir, L.; Amlouk, A.; Elaloui, E.; Saadoun, M.; Pierre, A.C. Preparation and optical characterization of transparent, microporous TiO2 xerogel monoliths. Mater. Sci. Eng. B 2008, 146, 69–73. [Google Scholar] [CrossRef]
- Fujita, K.; Konishi, J.; Nakanishi, K.; Hirao, K. Strong light scattering in macroporous TiO2 monoliths induced by phase separation. Appl. Phys. Lett. 2004, 85, 5595–5597. [Google Scholar] [CrossRef]
- Livage, J.; Henry, M.; Sanchez, C. Sol-gel chemistry of transition metal oxides. Prog. Solid State Chem. 1988, 18, 259–341. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science, The Physics and Chemistry of Sol-Gel Processing; Academic Press Inc.: New York, NY, USA, 1990. [Google Scholar]
- Bellayer, S.; Viau, L.; Tebby, Z.; Toupance, T.; Le Bideau, J.; Vioux, A. Immobilization of ionic liquids in translucent tin dioxide monoliths by sol-gel processing. Dalton Trans. 2009, 1307–1313. [Google Scholar] [CrossRef]
- Adachi, T.; Sakka, S. Preparation of monolithic silica gel and glass by the sol-gel method using N, N-dimethylformamide. J. Mater. Sci. 1987, 22, 4407–4410. [Google Scholar] [CrossRef]
- Adachi, T.; Sakka, S. The role of N, N - dimethylformamide, a DCCA, in the formation of silica-gel monoliths by sol-gel method. J. Non-Cryst. Solids 1988, 99, 118–128. [Google Scholar] [CrossRef]
- Wolfrum, S.M. Drying and sintering of Al2O3 compacts made by solgel processsing. J. Mater. Sci. Lett. 1987, 6, 706–708. [Google Scholar] [CrossRef]
- Bruin, S.; Luyben, K.C.A.M. Advances in Drying; Hemisphere: New York, NY, USA, 1980; Volume 1, pp. 217–267. [Google Scholar]
- Clifford, T.; Williams, J.R. Supercritical Fluid Methods and Protocols; Humana Press: Totowa, NJ, USA, 2000. [Google Scholar]
- Yoda, S.; Ohshima, S. Supercritical drying media modification for silica aerogel preparation. J. Non-Cryst. Solids 1999, 248, 224–234. [Google Scholar] [CrossRef]
- Yao, N.; Cao, S.L.; Yeung, K.L. Mesoporous TiO2-SiO2 aerogels with hierarchal pore structures. Microporous Mesoporous Mater. 2009, 117, 570–579. [Google Scholar] [CrossRef]
- Nakanishi, K.; Soga, N. Phase separation in silica sol-gel system containing poly(ethylene oxide) .2. Effects of molecular weight and temperature. Bull. Chem. Soc. Jpn. 1997, 70, 587–592. [Google Scholar] [CrossRef]
- Nakanishi, K. Pore Structure Control of Silica Gels Based on Phase Separation. J. Porous Mater. 1997, 4, 67–112. [Google Scholar] [CrossRef]
- Nishihara, H.; Mukai, S.R.; Fujii, Y.; Tago, T.; Masuda, T.; Tamon, H. Preparation of monolithic SiO2-Al2O3 cryogels with inter-connected macropores through ice templating. J. Mater. Chem. 2006, 16, 3231–3236. [Google Scholar] [CrossRef]
- Carbajo, M.C.; Lopez, C.; Gomez, A.; Enciso, E.; Torralvo, M.J. Micro/nano-structural properties of imprinted macroporous titania and zirconia. J. Mater. Chem. 2003, 13, 2311–2316. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, H.; Zhao, C.Y.; Liu, Y. Macroporous alumina monoliths prepared by filling polymer foams with alumina hydrosols. J. Mater. Sci. 2009, 44, 931–938. [Google Scholar] [CrossRef]
- Holland, B.T.; Blanford, C.F.; Stein, A. Synthesis of macroporous minerals with highly ordered three-dimensional arrays of spheroidal voids. Science 1998, 281, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.H.; Toepke, M.W.; Shanks, B.H. Surfactant-assisted synthesis of alumina with hierarchical nanopores. Adv. Funct. Mater. 2003, 13, 61–65. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Ren, T.Z.; Su, B.L. Hierarchically mesostructured titania materials with an unusual interior macroporous structure. Adv. Mater. 2003, 15, 1462–1465. [Google Scholar] [CrossRef]
- Grosso, D.; Illia, G.; Crepaldi, E.L.; Charleux, B.; Sanchez, C. Nanocrystalline transition-metal oxide spheres with controlled multi-scale porosity. Adv. Funct. Mater. 2003, 13, 37–42. [Google Scholar] [CrossRef]
- Chen, H.R.; Gu, J.L.; Shi, J.L.; Liu, Z.C.; Gao, J.H.; Ruan, M.L.; Yan, D.S. A composite surfactant route for the synthesis of thermally stable and hierarchically porous zirconia with a nanocrystallized framework. Adv. Mater. 2005, 17, 2010–2014. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Yang, H.F.; Zhao, D.Y. Synthesis of replica mesostructures by the nanocasting strategy. J. Mater. Chem. 2005, 15, 1217–1231. [Google Scholar]
- Taguchi, A.; Smatt, J.H.; Linden, M. Carbon monoliths possessing a hierarchical, fully interconnected porosity. Adv. Mater. 2003, 15, 1209–1211. [Google Scholar] [CrossRef]
- Lu, A.H.; Schuth, F. Nanocasting: A versatile strategy for creating nanostructured porous materials. Adv. Mater. 2006, 18, 1793–1805. [Google Scholar] [CrossRef]
- Smatt, J.H.; Weidenthaler, C.; Rosenholm, J.B.; Linden, M. Hierarchically porous metal oxide monoliths prepared by the nanocasting route. Chem. Mater. 2006, 18, 1443–1450. [Google Scholar] [CrossRef]
- Li, W.C.; Lu, A.H.; Schuth, F. Preparation of monolithic carbon aerogels and investigation of their pore interconnectivity by a nanocasting pathway. Chem. Mater. 2005, 17, 3620–3626. [Google Scholar] [CrossRef]
- Tong, Y.C.; Zhao, T.B.; Li, F.Y.; Wang, Y. Synthesis of monolithic zeolite beta with hierarchical porosity using carbon as a transitional template. Chem. Mater. 2006, 18, 4218–4220. [Google Scholar] [CrossRef]
- Tiemann, M. Repeated Templating. Chem. Mater. 2007, 20, 961–971. [Google Scholar] [CrossRef]
- Han, Y.J.; Kim, J.M.; Stucky, G.D. Preparation of noble metal nanowires using hexagonal mesoporous silica SBA-15. Chem. Mater. 2000, 12, 2068–2069. [Google Scholar] [CrossRef]
- Dong, A.G.; Wang, Y.J.; Wang, D.J.; Yang, W.L.; Zhang, Y.H.; Ren, N.; Gao, Z.; Tang, Y. Fabrication of hollow zeolite microcapsules with tailored shapes and functionalized interiors. Microporous Mesoporous Mater. 2003, 64, 69–81. [Google Scholar] [CrossRef]
- Yoldas, B.E. Hydrolysis of aluminium alkoxides and bayerite conversion. J. Appl. Chem. Biotechnol. 1973, 23, 803–809. [Google Scholar] [CrossRef]
- Yoldas, B.E. Alumina gels that form porous transparent Al2O3. J. Mater. Sci. 1975, 10, 1856–1860. [Google Scholar] [CrossRef]
- Pierre, A.; Begag, R.; Pajonk, G. Structure and texture of alumina aerogel monoliths made by complexation with ethyl acetoacetate. J. Mater. Sci. 1999, 34, 4937–4944. [Google Scholar] [CrossRef]
- Nass, R.; Schmidt, H. Synthesis of an alumina coating from chelated aluminum alkoxides. J. Non-Cryst. Solids 1990, 121, 329–333. [Google Scholar] [CrossRef]
- Heinrich, T.; Raether, F.; Marsmann, H. Growth and structure of single-phase mullite gels from chelated aluminum alkoxides and alkoxysilanes. J. Non-Cryst. Solids 1994, 168, 14–22. [Google Scholar] [CrossRef]
- Mizushima, Y.; Hori, M. Alumina aerogel for support of a methane combustion catalyst. Appl. Catal. A 1992, 88, 137–148. [Google Scholar] [CrossRef]
- Mizushima, Y.; Hori, M. Preparation of heat resistant alumina aerogels. J. Mater. Res. 1993, 8, 2993–2999. [Google Scholar] [CrossRef]
- Mizushima, Y.; Hori, M. Properties of alumina aerogels prepared under different conditions. J. Non-Cryst. Solids 1994, 167, 1–8. [Google Scholar] [CrossRef]
- Horiuchi, T.; Osaki, T.; Sugiyama, T.; Masuda, H.; Horio, M.; Suzuki, K.; Mori, T.; Sago, T. High-surface-area alumina aerogel at elevated-temperatures. J. Chem. Soc. Faraday Trans. 1994, 90, 2573–2578. [Google Scholar] [CrossRef]
- Keysar, S.; DeHazan, Y.; Cohen, Y.; Aboud, T.; Grader, G.S. Particle aggregation in alumina aerogels. J. Mater. Res. 1997, 12, 430–433. [Google Scholar] [CrossRef]
- Keysar, S.; Shter, G.E.; deHazan, Y.; Cohen, Y.; Grader, G.S. Heat treatment of alumina aerogels. Chem. Mater. 1997, 9, 2464–2467. [Google Scholar] [CrossRef]
- Grader, G.S.; Rifkin, Y.; Cohen, Y.; Keysar, S. Preparation of alumina aerogel films by low temperature CO2 supercritical drying process. J. Sol-Gel Sci. Technol. 1997, 8, 825–829. [Google Scholar]
- Li, L.L.; Duan, W.T.; Yuan, Q.; Li, Z.X.; Duan, H.H.; Yan, C.H. Hierarchical gamma-Al2O3 monoliths with highly ordered 2D hexagonal mesopores in macroporous walls. Chem. Commun. 2009, 6174–6176. [Google Scholar] [CrossRef]
- Macedo, M.I.F.; Osawa, C.C.; Bertran, C.A. Sol-gel synthesis of transparent alumina gel and pure gamma alumina by urea hydrolysis of aluminum nitrate. J. Sol-Gel Sci. Technol. 2004, 30, 135–140. [Google Scholar] [CrossRef]
- Music, S.; Dragcevic, D.; Popovic, S.; Vdovic, N. Chemical and microstructural properties of Al-oxide phases obtained from AlCl3 solutions in alkaline medium. Mater. Chem. Phys. 1999, 59, 12–19. [Google Scholar] [CrossRef]
- Bai, P.; Wu, P.P.; Yan, Z.F.; Zhao, X.S. A reverse cation-anion double hydrolysis approach to the synthesis of mesoporous gamma-Al2O3 with a bimodal pore size distribution. Microporous Mesoporous Mater. 2009, 118, 288–295. [Google Scholar] [CrossRef]
- Baumann, T.F.; Gash, A.E.; Chinn, S.C.; Sawvel, A.M.; Maxwell, R.S.; Satcher, J.H. Synthesis of high-surface-area alumina aerogels without the use of alkoxide precursors. Chem. Mater. 2005, 17, 395–401. [Google Scholar] [CrossRef]
- Tokudome, Y.; Fujita, K.; Nakanishi, K.; Miura, K.; Hirao, K. Synthesis of monolithic Al2O3 with well-defined macropores and mesostructured skeletons via the sol-gel process accompanied by phase separation. Chem. Mater. 2007, 19, 3393–3398. [Google Scholar] [CrossRef]
- Tokudome, Y.; Nakanishi, K.; Kanamori, K.; Fujita, K.; Akamatsu, H.; Hanada, T. Structural characterization of hierarchically porous alumina aerogel and xerogel monoliths. J. Colloid Interface Sci. 2009, 338, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Tokudome, Y.; Nakanishi, K.; Hanada, T. Effect of La addition on thermal microstructural evolution of macroporous alumina monolith prepared from ionic precursors. J. Ceram. Soc. Jpn. 2009, 117, 351–355. [Google Scholar] [CrossRef]
- Thiruchitrambalam, M.; Palkar, V.R.; Gopinathan, V. Hydrolysis of aluminium metal and sol-gel processing of nano alumina. Mater. Lett. 2004, 58, 3063–3066. [Google Scholar] [CrossRef]
- Gieselmann, M.J.; Anderson, M.A. Effect of ionic-strength on boehmite hydrogel formation. J. Am. Ceram. Soc. 1989, 72, 980–985. [Google Scholar] [CrossRef]
- Kiovsky, J.R.; Meacham, J.W. High surface area alumina bodies. U.S.Patent 3,945,945, 1976. [Google Scholar]
- Kwon, S.; Messing, G.L. Dry Pressing Boehmite Gels for the Fabrication of Monolithic α-Al2O3. J. Sol-Gel Sci. Technol. 1997, 9, 53–64. [Google Scholar]
- Zhang, Y.; Liang, H.; Zhao, C.; Liu, Y. Macroporous alumina monoliths prepared by filling polymer foams with alumina hydrosols. J. Mater. Sci. 2009, 44, 931–938. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel-cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Yoshimura, M. Phase stability of zirconia. Am. Ceram. Soc. Bull. 1988, 67, 1950–1955. [Google Scholar]
- Mondal, A.; Ram, S. Enhanced phase stability and photoluminescence of Eu3+ modified t-ZrO2 nanoparticles. J. Am. Ceram. Soc. 2008, 91, 329–332. [Google Scholar] [CrossRef]
- Hisbergues, M.; Vendeville, S.; Vendeville, P. Zirconia: Established Facts and Perspectives for a Biomaterial in Dental Implantology. J. Biomed. Mater. Res. B 2009, 88B, 519–529. [Google Scholar] [CrossRef]
- Andreiotelli, M.; Wenz, H.J.; Kohal, R.J. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clin. Oral Implant. Res. 2009, 20, 32–47. [Google Scholar] [CrossRef]
- Wenz, H.J.; Bartsch, J.; Wolfart, S.; Kern, M. Osseointegration and clinical success of zirconia dental implants: A systematic review. Int. J. Prosthodont. 2008, 21, 27–36. [Google Scholar] [PubMed]
- Sun, C.W.; Stimming, U. Recent anode advances in solid oxide fuel cells. J. Power Sources 2007, 171, 247–260. [Google Scholar] [CrossRef]
- Youn, M.H.; Seo, J.G.; Jung, J.C.; Park, S.; Park, D.R.; Lee, S.-B.; Song, I.K. Hydrogen production by auto-thermal reforming of ethanol over Ni catalyst supported on ZrO2 prepared by a sol-gel method: Effect of H2O/P123 mass ratio in the preparation of ZrO2. Catal. Today 2009, 146, 57–62. [Google Scholar] [CrossRef]
- Youn, M.H.; Seo, J.G.; Cho, K.M.; Park, S.; Park, D.R.; Jung, J.C.; Song, I.K. Hydrogen production by auto-thermal reforming of ethanol over nickel catalysts supported on Ce-modified mesoporous zirconia: Effect of Ce/Zr molar ratio. Int. J. Hydrogen Energy 2008, 33, 5052–5059. [Google Scholar] [CrossRef]
- Gaertner, C.A.; Serrano-Ruiz, J.C.; Braden, D.J.; Dumesic, J.A. Catalytic coupling of carboxylic acids by ketonization as a processing step in biomass conversion. J. Catal. 2009, 266, 71–78. [Google Scholar] [CrossRef]
- Boaro, M.; Vicario, M.; Llorca, J.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. A comparative study of water gas shift reaction over gold and platinum supported on ZrO2 and CeO2-ZrO2. Appl. Catal. B 2009, 88, 272–282. [Google Scholar] [CrossRef]
- Zizkovský, V.; Kucera, R.; Klimes, J. Potential employment of non-silica-based stationary phases in pharmaceutical analysis. J. Pharm. Biomed. Anal. 2007, 44, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Blin, J.L.; Leonard, A.; Yuan, Z.Y.; Gigot, L.; Vantomme, A.; Cheetham, A.K.; Su, B.L. Hierarchically mesoporous/macroporous metal oxides templated from polyethylene oxide surfactant assemblies. Angew. Chem. Int. Ed. 2003, 42, 2872–2875. [Google Scholar] [CrossRef]
- Wan, Y.; Ma, J.; Zhou, W.; Zhu, Y.; Song, X.; Li, H. Preparation of titania-zirconia composite aerogel material by sol-gel combined with supercritical fluid drying. Appl. Catal. A 2004, 277, 55–59. [Google Scholar] [CrossRef]
- Quaschning, V.; Auroux, A.; Deutsch, J.; Lieske, H.; Kemnitz, E. Microcalorimetric and Catalytic Studies on Sulfated Zirconia Catalysts of Different Preparations. J. Catal. 2001, 203, 426–433. [Google Scholar] [CrossRef]
- Konishi, J.; Fujita, K.; Oiwa, S.; Nakanishi, K.; Hirao, K. Crystalline ZrO2 monoliths with well-defined macropores and mesostructured skeletons prepared by combining the alkoxy-derived sol-gel process accompanied by phase separation and the solvothermal process. Chem. Mater. 2008, 20, 2165–2173. [Google Scholar] [CrossRef]
- Chervin, C.N.; Clapsaddle, B.J.; Chiu, H.W.; Gash, A.E.; Satcher, J.H.; Kauzlarich, S.M. Aerogel Synthesis of Yttria-Stabilized Zirconia by a Non-Alkoxide Sol-Gel Route. Chem. Mater. 2005, 17, 3345–3351. [Google Scholar] [CrossRef]
- Ram, S.; Mondal, A. Synthesis of monolithic nanoparticulate ZrO2 in a new polymorph of orthorhombic crystal structure at ambient pressure. Phys. Status Solidi A 2004, 201, 696–707. [Google Scholar] [CrossRef]
- Mondal, A.; Ram, S. Monolithic t-ZrO2 nanopowder through a ZrO(OH)2*xH2O polymer precursor. J. Am. Ceram. Soc. 2004, 87, 2187–2194. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Chen, D.R.; Jiao, X.L. Zirconia aerogels with high surface area derived from sols prepared by electrolyzing zirconium oxychloride solution: Comparison of aerogels prepared by freeze-drying and supercritical CO2(l) extraction. J. Phys. Chem. C 2007, 111, 18738–18743. [Google Scholar] [CrossRef]
- Fujihira, M.; Satoh, Y.; Osa, T. Heterogeneous photocatalytic oxidation of aromatic compounds on TiO2. Nature 1981, 293, 206–208. [Google Scholar] [CrossRef]
- Gopel, W.; Kirner, U.; Wiemhofer, H.D.; Rocker, G. Surface and bulk properties of TiO2 in relation to sensor applications. Solid State Ionics 1988, 28, 1423–1430. [Google Scholar] [CrossRef]
- Buchmeiser, M.R. New synthetic ways for the preparation of high-performance liquid chromatography supports. J. Chromatogr. A 2001, 918, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Yamabi, S.; Imai, H. Crystal Phase Control for Titanium Dioxide Films by Direct Deposition in Aqueous Solutions. Chem. Mater. 2002, 14, 609–614. [Google Scholar] [CrossRef]
- Kho, Y.K.; Iwase, A.; Teoh, W.Y.; Madler, L.; Kudo, A.; Amal, R. Photocatalytic H-2 Evolution over TiO2 Nanoparticles. The Synergistic Effect of Anatase and Rutile. J. Phys. Chem. C 114, 2821–2829.
- Konishi, J.; Fujita, K.; Nakanishi, K.; Hirao, K. Monolithic TiO2 with controlled multiscale porosity via a template-free sol-gel process accompanied by phase separation. Chem. Mater. 2006, 18, 6069–6074. [Google Scholar] [CrossRef]
- Konishi, J.; Fujita, K.; Nakanishi, K.; Hirao, K. Phase-separation-induced Titania monoliths with well-derined macropores and mesostructured framework from colloid-derived sol-gel systems. Chem. Mater. 2006, 18, 864–866. [Google Scholar] [CrossRef]
- Konishi, J.; Fujita, K.; Nakanishi, K.; Hirao, K.; Morisato, K.; Miyazaki, S.; Ohira, M. Sol-gel synthesis of macro-mesoporous titania monoliths and their applications to chromatographic separation media for organophosphate compounds. J. Chromatogr. A 2009, 1216, 7375–7383. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yi, Y.Y.; Brennan, J.D.; Brook, M.A. Development of macroporous Titania monoliths using a biocompatible method. Part 1: Material fabrication and characterization. Chem. Mater. 2006, 18, 5326–5335. [Google Scholar] [CrossRef]
- Shchipunov, Y.; Postnova, I. One-pot biomimetic synthesis of monolithic titania through mineralization of polysaccharide. Colloids Surf. B 2009, 74, 172–177. [Google Scholar] [CrossRef]
- Gill, S.K.; Hope-Weeks, L.J. Monolithic aerogels of silver modified cadmium sulfide colloids. Chem. Commun. 2009, 4384–4386. [Google Scholar] [CrossRef]
- Smatt, J.H.; Spliethoff, B.; Rosenholm, J.B.; Linden, M. Hierachically porous nanocrystalline cobalt oxide monoliths through nanocasting. Chem. Commun. 2004, 2188–2189. [Google Scholar] [CrossRef]
- Dong, W.; Yen, S.P.; Paik, J.A.; Sakamoto, J. The Role of Acetic Acid and Glycerol in the Synthesis of Amorphous MgO Aerogels. J. Am. Ceram. Soc. 2009, 92, 1011–1016. [Google Scholar] [CrossRef]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H.; Poco, J.F.; Hrubesh, L.W.; Simpson, R.L. Use of epoxides in the sol-gel synthesis of porous iron(III) oxide monoliths from Fe(III) salts. Chem. Mater. 2001, 13, 999–1007. [Google Scholar] [CrossRef]
- Gash, A.E.; Satcher, J.H.; Simpson, R.L. Strong akaganeite aerogel monoliths using epoxides: Synthesis and characterization. Chem. Mater. 2003, 15, 3268–3275. [Google Scholar] [CrossRef]
- Panda, M.; Rajamathi, M.; Seshadri, R. A template-free, combustion-chemical route to macroporous nickel monoliths displaying a hierarchy of pore sizes. Chem. Mater. 2002, 14, 4762–4767. [Google Scholar] [CrossRef]
- Gao, Y.P.; Sisk, C.N.; Hope-Weeks, L.J. A sol-gel route to synthesize monolithic zinc oxide aerogels. Chem. Mater. 2007, 19, 6007–6011. [Google Scholar] [CrossRef]
- Richter, K.; Backer, T.; Mudring, A.V. Facile, environmentally friendly fabrication of porous silver monoliths using the ionic liquid N-(2-hydroxyethyl)ammonium formate. Chem. Commun. 2009, 301–303. [Google Scholar] [CrossRef]
- Wang, X.Q.; Bozhilov, K.N.; Feng, P.Y. Facile preparation of hierarchically porous carbon monoliths with well-ordered mesostructures. Chem. Mater. 2006, 18, 6373–6381. [Google Scholar] [CrossRef]
- Jaroniec, M.; Choma, J.; Gorka, J.; Zawislak, A. Colloidal silica templating synthesis of carbonaceous monoliths assuring formation of uniform spherical mesopores and incorporation of inorganic nanoparticles. Chem. Mater. 2008, 20, 1069–1075. [Google Scholar] [CrossRef]
- Xu, L.Y.; Shi, Z.G.; Feng, Y.Q. Preparation of a carbon monolith with bimodal perfusion pores. Microporous Mesoporous Mater. 2008, 115, 618–623. [Google Scholar] [CrossRef]
- Sonnenburg, K.; Adelhelm, P.; Antonietti, M.; Smarsly, B.; Noske, R.; Strauch, P. Synthesis and characterization of SiC materials with hierarchical porosity obtained by replication techniques. Phys. Chem. Chem. Phys. 2006, 8, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Comotti, M.; Lu, A.H.; Schuth, F. Nanocast mesoporous MgAl2O4 spinel monoliths as support for highly active gold CO oxidation catalyst. Chem. Commun. 2006, 1772–1774. [Google Scholar] [CrossRef]
- Minakuchi, H.; Nakanishi, K.; Soga, N.; Ishizuka, N.; Tanaka, N. Octadecylsilylated porous silica rods as separation media for reversed-phase liquid chromatography. Anal. Chem. 1996, 68, 3498–3501. [Google Scholar] [CrossRef] [PubMed]
- Minakuchi, H.; Nakanishi, K.; Soga, N.; Ishizuka, N.; Tanaka, N. Effect of domain size on the performance of octadecylsilylated continuous porous silica columns in reversed-phase liquid chromatography. J. Chromatogr. A 1998, 797, 121–131. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gaweł, B.; Gaweł, K.; Øye, G. Sol-Gel Synthesis of Non-Silica Monolithic Materials. Materials 2010, 3, 2815-2833. https://doi.org/10.3390/ma3042815
Gaweł B, Gaweł K, Øye G. Sol-Gel Synthesis of Non-Silica Monolithic Materials. Materials. 2010; 3(4):2815-2833. https://doi.org/10.3390/ma3042815
Chicago/Turabian StyleGaweł, Bartłomiej, Kamila Gaweł, and Gisle Øye. 2010. "Sol-Gel Synthesis of Non-Silica Monolithic Materials" Materials 3, no. 4: 2815-2833. https://doi.org/10.3390/ma3042815
APA StyleGaweł, B., Gaweł, K., & Øye, G. (2010). Sol-Gel Synthesis of Non-Silica Monolithic Materials. Materials, 3(4), 2815-2833. https://doi.org/10.3390/ma3042815




