Dendritic Cell-Induced Th1 and Th17 Cell Differentiation for Cancer Therapy
Abstract
:1. CD4+ T-Cell Differentiation
2. Requirements for Differentiation of Th17 Cells
| Cytokines | Effect on human Th17 cell differentiation |
|---|---|
| IL-1β | Positive effect on Th17 cell differentiation |
| Acosta-Rodriquez; Nature Immunology, 2007 [22] | |
| van Beelen; Immunity, 2007 [29] | |
| Manel; Nature Immunology, 2008 [32] | |
| Zielinski; Nature Letters, 2012 [33] | |
| Kryczek; Blood, 2009 [34] | |
| Wilson; Nature Immunology, 2007 [35] | |
| Volpe; Nature Immunology, 2008 [36] | |
| IL-6 | Positive effect on Th17 cell differentiation |
| Acosta-Rodriquez; Nature Immunology, 2007 [22] | |
| Manel; Nature Immunology, 2008 [32] | |
| Zielinski; Nature Letters, 2012 [33] | |
| Volpe; Nature Immunology, 2008 [36] | |
| Not necessary for Th17 cell differentiation | |
| van Beelen; Immunity, 2007 [29] | |
| Kryczek; Blood, 2009 [34] | |
| Wilson; Nature Immunology, 2007 [35] | |
| Evans; Proc. Natl. Acad. Sci., 2007 [37] | |
| IL-23 | Positive effect on Th17 cell differentiation |
| van Beelen; Immunity, 2007 [29] | |
| Manel; Nature Immunology, 2008 [32] | |
| Zielinski; Nature Letters 2012 [33] | |
| Kryczek; Blood, 2009 [34] | |
| Wilson; Nature Immunology, 2007 [35] | |
| Volpe; Nature Immunology, 2008 [36] | |
| Necessary for effector function of Th17, but not for differentiation | |
| Veldhoen; Immunity, 2006 [38] | |
| Elson; Gastroenterology, 2007 [39] | |
| TGF-β | Positive effect on Th17 cell differentiation |
| Manel; Nature Immunology, 2007 [32] | |
| Volpe; Nature Immunology, 2008 [36] | |
| Negative effect on Th17 cell differentiation | |
| Acosta-Rodriquez; Nature Immunology, 2007 [22] | |
| Wilson; Nature Immunology, 2007 [35] | |
| Evans; Proceeding of the National Acadamy of Science USA, 2007 [37] | |
| Not necessary for Th17 cell differentiation | |
| van Beelen; Immunity, 2007 [29] | |
| Kryczek; Blood, 2009 [34] |
2.1. IL-23
2.2. Transforming Growth Factor-β
2.3. Interleukin-6
2.4. IL-1β
2.5. Tc17 Cell Differentiation
3. Th1 and Th17 Cells in Autoimmunity and Cancer
3.1. Autoimmunity
3.2. Cancer
| Pro-tumor |
|
| Anti-tumor |
|
4. Dendritic Cells for Cancer Immunotherapy
4.1. Toll-Like Receptor (TLR) Agonists as Maturation Agents for DC1 and DC17s and Polarization of T-Cell Responses
4.2. Use of Prostaglandin E2 as a DC Maturation Agent
4.3. Interleukin-4
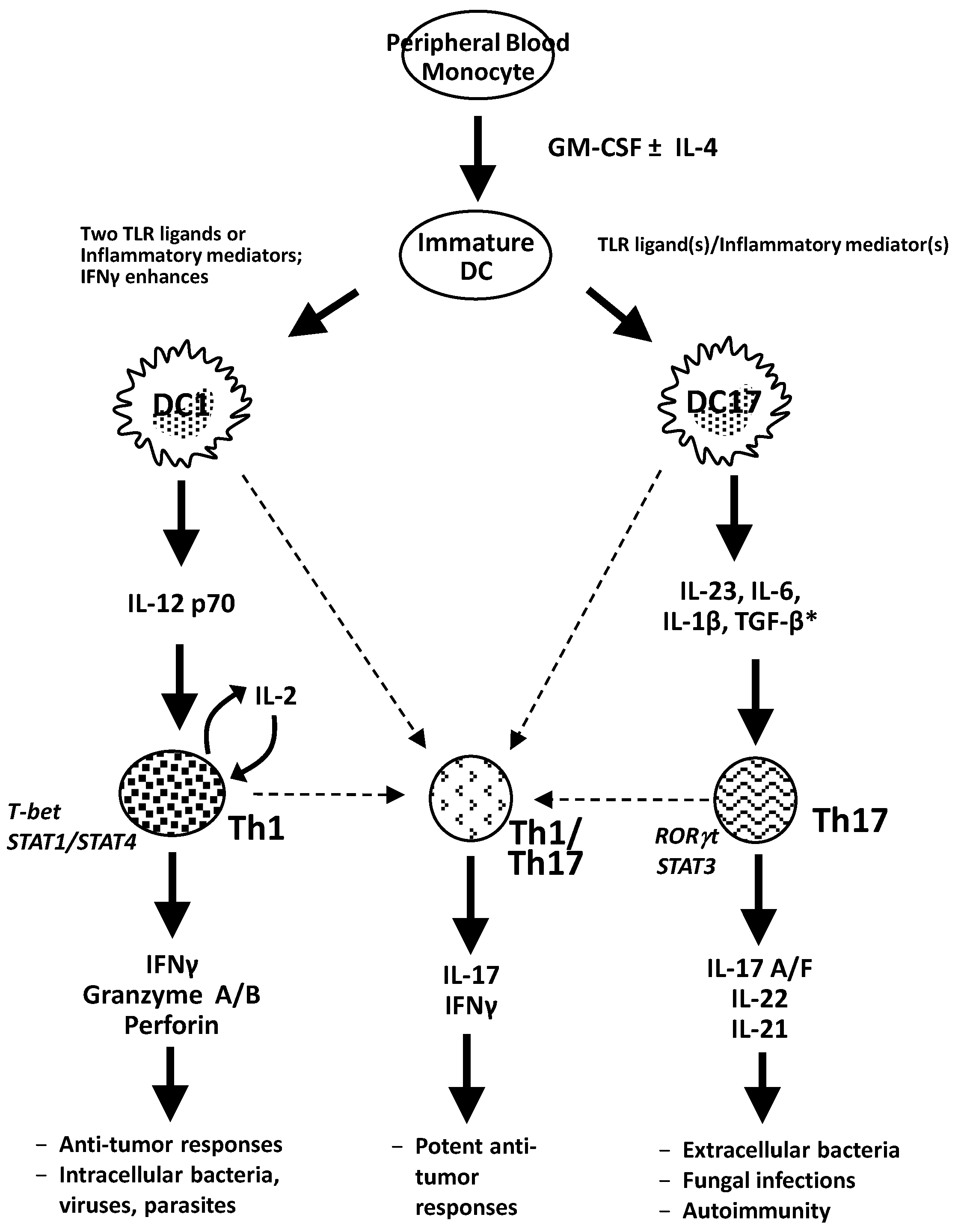
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- Steinman, R.M.; Young, J.W. Signals arising from antigen-presenting cells. Curr. Opin. Immun. 1991, 3, 361–372. [Google Scholar] [CrossRef]
- Kalinski, P.; Hilkens, C.M.; Wierenga, E.A.; Kapsenberg, M.L. T-cell priming by type-1 and type-2 polarized dendritic cells: The concept of a third signal. Immun. Today 1999, 20, 561–567. [Google Scholar] [CrossRef]
- De Jong, E.C.; Smits, H.H.; Kapsenberg, M.L. Dendritic cell-mediated T-cell polarization. Springer Semin. Immunopathol. 2005, 26, 289–307. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Ann. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Sad, S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immun. Today 1996, 17, 138–146. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Fili, L.; Ferri, S.; Frosali, F.; et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007, 204, 1849–1861. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+ T-cells: Differentiation and functions. Clin. Develop. Immun. 2012, 2012. [Google Scholar] [CrossRef]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef]
- Zheng, W.; Flavell, R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T-cells. Cell 1997, 89, 587–596. [Google Scholar] [CrossRef]
- Van Panhuys, N.; Prout, M.; Forbes, E.; Min, B.; Paul, W.E.; Le Gros, G. Basophils are the major producers of IL-4 during primary helminth infection. J. Immunol. 2011, 186, 2719–2728. [Google Scholar] [CrossRef]
- Trinchieri, G.; Wysocka, M.; D’Andrea, A.; Rengaraju, M.; Aste-Amezaga, M.; Kubin, M.; Valiente, N.M.; Chehimi, J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog. Growth Factor Res. 1992, 4, 355–368. [Google Scholar] [CrossRef]
- Loser, K.; Beissert, S. Regulatory T-cells: Banned cells for decades. J. Invest. Dermatol. 2012, 132, 864–871. [Google Scholar] [CrossRef]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T-cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef]
- Pene, J.; Chevalier, S.; Preisser, L.; Venereau, E.; Guilleux, M.H.; Ghannam, S.; Moles, J.P.; Danger, Y.; Ravon, E.; Lesaux, S.; et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J. Immunol. 2008, 180, 7423–7430. [Google Scholar]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T-cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef]
- Numasaki, M.; Fukushi, J.; Ono, M.; Narula, S.K.; Zavodny, P.J.; Kudo, T.; Robbins, P.D.; Tahara, H.; Lotze, M.T. Interleukin-17 promotes angiogenesis and tumor growth. Blood 2003, 101, 2620–2627. [Google Scholar] [CrossRef]
- Elser, B.; Lohoff, M.; Kock, S.; Giaisi, M.; Kirchoff, S.; Krammer, P.H.; Li-Weber, M. IFN-gamma represses IL-4 expression via IRF-1 and IRF-2. Immunity 2002, 17, 703–712. [Google Scholar] [CrossRef]
- Szabo, S.J.; Dighe, A.S.; Gubler, U.; Murphy, K.M. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997, 185, 817–824. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Vieira, P.; Mosmann, T.R.; Howard, M.; Moore, K.W.; O’Garra, A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991, 146, 3444–3451. [Google Scholar]
- Hoeve, M.A.; Savage, N.D.; de Boer, T.; Langenberg, D.M.; de Waal Malefyt, R.; Ottenhoff, T.H.; Verreck, F.A. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T-cells. Eur. J. Immun. 2006, 36, 661–670. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producinghuman T helper cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar]
- Ouyang, W.; Ranganath, S.H.; Weindel, K.; Bhattacharya, D.; Murphy, T.L.; Sha, W.C.; Murphy, K.M. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity 1998, 9, 745–755. [Google Scholar] [CrossRef]
- Mathur, A.N.; Chang, H.C.; Zisoulis, D.G.; Kapur, R.; Belladonna, M.L.; Kansas, G.S.; Kaplan, M.H. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood 2006, 108, 1595–1601. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fitz, L.; Ryan, M.; Hewick, R.M.; Clark, S.C.; Chan, S.; Loudon, R.; Sherman, F.; Perussia, B.; Trinchieri, G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989, 170, 827–845. [Google Scholar] [CrossRef]
- Manetti, R.; Parronchi, P.; Giudizi, M.G.; Piccinni, M.P.; Maggi, E.; Trinchieri, G.; Romagnani, S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 1993, 177, 1199–1204. [Google Scholar] [CrossRef]
- Chan, S.H.; Perussia, B.; Gupta, J.W.; Kobayashi, M.; Pospisil, M.; Young, H.A.; Wolf, S.F.; Young, D.; Clark, S.C.; Trinchieri, G. Induction of interferon gamma production by natural killer cell stimulatory factor: Characterization of the responder cells and synergy with other inducers. J. Exp. Med. 1991, 173, 869–879. [Google Scholar] [CrossRef]
- Cosmi, L.; de Palma, R.; Santarlasci, V.; Maggi, L.; Capone, M.; Frosali, F.; Rodolico, G.; Querci, V.; Abbate, G.; Angeli, R.; et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T-cell precursor. J. Exp. Med. 2008, 205, 1903–1916. [Google Scholar] [CrossRef]
- Van Beelen, A.J.; Zelinkova, Z.; Taanman-Kueter, E.W.; Muller, F.J.; Hommes, D.W.; Zaat, S.A.J.; Kapsenberg, M.L.; de Jong, E.C. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T-cells. Immunity 2007, 27, 660–669. [Google Scholar] [CrossRef]
- Maggi, L.; Santarlasci, V.; Capone, M.; Peired, A.; Frosali, F.; Crome, S.Q.; Querci, V.; Fambrini, M.; Liotta, F.; Levings, M.K.; et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur. J. Immunol. 2010, 40, 2174–2181. [Google Scholar] [CrossRef]
- Crome, S.Q.; Wang, A.Y.; Kang, C.Y.; Levings, M.K. The role of retinoic acid-related orphan receptor variant 2 and IL-17 in the development and function of human CD4+ T-cells. Eur. J. Immun. 2009, 39, 1480–1493. [Google Scholar] [CrossRef]
- Manel, N.; Unutmaz, D.; Littman, D.R. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008, 9, 641–649. [Google Scholar] [CrossRef]
- Zielinski, C.E.; Mele, F.; Aschenbrenner, D.; Jarrossay, D.; Ronchi, F.; Gattorno, M.; Monticelli, S.; Lanzavecchia, A.; Sallusto, F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 2012, 484, 514–518. [Google Scholar] [CrossRef]
- Kryczek, I.; Banerjee, M.; Cheng, P.; Vatan, L.; Szeliga, W.; Wei, S.; Huang, E.; Finlayson, E.; Simeone, D.; Welling, T.H.; et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009, 114, 1141–1149. [Google Scholar] [CrossRef]
- Wilson, N.J.; Boniface, K.; Chan, J.R.; McKenzie, B.S.; Blumenschein, W.M.; Mattson, J.D.; Basham, B.; Smith, K.; Chen, T.; Morel, F.; et al. Development, cytokine profile and function of human interleukin 17-producing helper T-cells. Nat. Immun. 2007, 8, 950–957. [Google Scholar]
- Volpe, E.; Servant, N.; Zollinger, R.; Bogiatzi, S.I.; Hupe, P.; Barillot, E.; Soumelis, V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 2008, 9, 650–657. [Google Scholar]
- Evans, H.G.; Suddason, T.; Jackson, I.; Taams, L.S.; Lord, G.M. Optimal induction of T helper 17 cells in humans requires T-cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc. Natl. Acad. Sci. USA 2007, 104, 17034–17039. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T-cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Elson, C.O.; Cong, Y.; Weaver, C.T.; Schoeb, T.R.; McClanahan, T.K.; Fick, R.B.; Kastelein, R.A. Monoclonal anti-interleukin 23 reverses active colitis in a T-cell-mediated model in mice. Gastroenterology 2007, 132, 2359–2370. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ghilardi, N.; Xie, M.H.; de Sauvage, F.J.; Gurney, A.L. Interleukin-23 promotes a distinct CD4 T-cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003, 278, 1910–1914. [Google Scholar]
- Murphy, C.A.; Langrish, C.L.; Chen, Y.; Blumenschein, W.; McClanahan, T.; Kastelein, R.A.; Sedgwick, J.D.; Cua, D.J. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003, 198, 1951–1957. [Google Scholar] [CrossRef]
- Mangan, P.R.; Harrington, L.E.; O’Quinn, D.B.; Helms, W.S.; Bullard, D.C.; Elson, C.O.; Hatton, R.D.; Wahl, S.M.; Schoeb, T.R.; Weaver, C.T. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 2006, 441, 231–234. [Google Scholar] [CrossRef]
- Pesce, B.; Soto, L.; Sabugo, F.; Wurmann, P.; Cuchacovich, M.; Lopez, M.N.; Sotelo, P.H.; Molina, M.C.; Aguillon, J.C.; Catalan, D. Effect of interleukin-6 receptor blockade on the balance between regulatory T-cells and T helper type 17 cells in rheumatoid arthritis patients. Clin. Exp. Immun. 2013, 171, 237–242. [Google Scholar] [CrossRef]
- Zhou, L.; Ivanov, I.I.; Spolski, R.; Min, R.; Shenderov, K.; Egawa, T.; Levy, D.E.; Leonard, W.J.; Littman, D.R. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007, 8, 967–974. [Google Scholar] [CrossRef]
- Hinrichs, C.S.; Kaiser, A.; Paulos, C.M.; Cassard, L.; Sanchez-Perez, L.; Heemskerk, B.; Wrzesinski, C.; Borman, Z.A.; Muranski, P.; Restifo, N.P. Type 17 CD8+ T-cells display enhanced antitumor immunity. Blood 2009, 144, 596–599. [Google Scholar]
- Huber, M.; Heink, S.; Grothe, H.; Guralnik, A.; Reinhard, K.; Elflein, K.; Hunig, T.; Mittrucker, H.W.; Brustle, A.; Kamradt, T.; et al. A Th17-like developmental process leads to CD8+ Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol. 2009, 39, 1716–1725. [Google Scholar] [CrossRef]
- Liu, S.J.; Tsai, J.P.; Shen, C.R.; Sher, Y.P.; Hsieh, C.L.; Yen, Y.C.; Chou, A.H.; Chang, S.R.; Hsiao, K.N.; Yu, F.K.; et al. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J. Leukocyte Biol. 2007, 82, 354–360. [Google Scholar] [CrossRef]
- Caruso, R.; Fina, D.; Paoluzi, O.A.; Del Vecchio Blanco, G.; Stolfi, C.; Rizzo, A.; Caprioli, F.; Sarra, M.; Andrei, F.; Fantini, M.C.; et al. IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. Eur. J. Immun. 2008, 38, 470–478. [Google Scholar] [CrossRef]
- Kuang, D.M.; Peng, C.; Zhao, Q.; Wu, Y.; Zhu, L.Y.; Wang, J.; Yin, X.Y.; Li, L.; Zheng, L. Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T-cells in hepatocellular carcinoma patients. J. Immun. 2010, 185, 1544–1549. [Google Scholar] [CrossRef]
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef]
- Cua, D.J.; Sherlock, J.; Chen, Y.; Murphy, C.A.; Joyce, B.; Seymour, B.; Lucian, L.; To, W.; Kwan, S.; Churakova, T.; et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 412, 744–748. [Google Scholar]
- Metawi, S.A.; Abbas, D.; Kamal, M.M.; Ibrahim, M.K. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clin. Rheumatol. 2011, 30, 1201–1207. [Google Scholar] [CrossRef]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef]
- Nakae, S.; Nambu, A.; Sudo, K.; Iwakura, Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003, 171, 6173–6177. [Google Scholar]
- Bush, K.A.; Farmer, K.M.; Walker, J.S.; Kirkham, B.W. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002, 46, 802–805. [Google Scholar] [CrossRef]
- Komiyama, Y.; Nakae, S.; Matsuki, T.; Nambu, A.; Ishigame, H.; Kakuta, S.; Sudo, K.; Iwakura, Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006, 177, 566–573. [Google Scholar]
- Schattner, E.J.; Mascarenhas, J.; Bishop, J.; Yoo, D.H.; Chadburn, A.; Crow, M.K.; Friedman, S.M. CD4+ T-cell induction of Fas-mediated apoptosis in Burkitt’s lymphoma B cells. Blood 1996, 88, 1375–1382. [Google Scholar]
- Thomas, W.D.; Hersey, P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T-cell killing of targeT-cells. J. Immunol. 1998, 161, 2195–2200. [Google Scholar]
- Echchakir, H.; Bagot, M.; Dorothee, G.; Martinvalet, D.; le Gouvello, S.; Boumsell, L.; Chouaib, S.; Bensussan, A.; Mami-Chouaib, F. Cutaneous T-cell lymphoma reactive CD4+ cytotoxic T lymphocyte clones display a Th1 cytokine profile and use a fas-independent pathway for specific tumor cell lysis. J. Invest. Dermatol. 2000, 115, 74–80. [Google Scholar] [CrossRef]
- Bourgeois, C.; Rocha, B.; Tanchot, C. A role for CD40 expression on CD8+ T-cells in the generation of CD8+ T-cell memory. Science 2002, 297, 2060–2063. [Google Scholar] [CrossRef]
- Janssen, E.M.; Lemmens, E.E.; Wolfe, T.; Christen, U.; von Herrath, M.G.; Schoenberger, S.P. CD4+ T-cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003, 421, 852–856. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, D.E.; Lim, S.Y.; Lee, S.H.; Kang, J.L.; Lee, S.J.; Benveniste, E.N.; Choi, Y.H. Loss of the promyelocytic leukemia protein in gastric cancer: Implications for IP-10 expression and tumor-infiltrating lymphocytes. PLoS One 2011, 6, e26264. [Google Scholar]
- Zhu, X.; Fallert-Junecko, B.A.; Fujita, M.; Ueda, R.; Kohanbash, G.; Kastenhuber, E.R.; McDonald, H.A.; Liu, Y.; Kalinski, P.; Reinhart, T.A.; et al. Poly-ICLC promotes the infiltration of effector T-cells into intracranial gliomas via induction of CXCL10 in IFN-alpha and IFN-gamma dependent manners. Cancer Immunol. Immunother. 2010, 59, 1401–1409. [Google Scholar] [CrossRef]
- Curtsinger, J.M.; Lins, D.C.; Johnson, C.M.; Mescher, M.F. Signal. 3 tolerant CD8 T-cells degranulate in response to antigen but lack granzyme B to mediate cytolysis. J. Immunol. 2005, 175, 4392–4399. [Google Scholar]
- Valenzuela, J.O.; Hammerbeck, C.D.; Mescher, M.F. Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T-cells. J. Immunol. 2005, 174, 600–604. [Google Scholar]
- Xu, S.; Koski, G.K.; Faries, M.; Bedrosian, I.; Mick, R.; Maeurer, M.; Cheever, M.A.; Cohen, P.A.; Czerniecki, B.J. Rapid high efficiency sensitization of CD8+ T-cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J. Immunol. 2003, 171, 2251–2261. [Google Scholar]
- Tartour, E.; Fossiez, F.; Joyeux, I.; Galinha, A.; Gey, A.; Claret, E.; Sastre-Garau, X.; Couturier, J.; Mosseri, V.; Vives, V.; et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999, 59, 3698–3704. [Google Scholar]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef]
- Chen, W.C.; Lai, Y.H.; Chen, H.Y.; Guo, H.R.; Su, I.J.; Chen, H.H. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology 2013, 63, 225–233. [Google Scholar] [CrossRef]
- Benevides, L.; Cardoso, C.R.; Tiezzi, D.G.; Marana, H.R.; Andrade, J.M.; Silva, J.S. Enrichment of regulatory T-cells in invasive breast tumor correlates with the upregulation of IL-17A expression and invasiveness of the tumor. Eur. J. Immun. 2013, 43, 1518–1528. [Google Scholar] [CrossRef]
- Qian, X.; Gu, L.; Ning, H.; Zhang, Y.; Hsueh, E.C.; Fu, M.; Hu, X.; Wei, L.; Hoft, D.F.; Liu, J. Increased Th17 Cells in the Tumor Microenvironment Is Mediated by IL-23 via Tumor-Secreted Prostaglandin E2. J. Immunol. 2013, 190, 5894–5902. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, H.; Deng, L.; Hou, J.; Shi, R.; Yao, M.; Gao, Y.; Yao, A.; Wang, X.; Yu, L.; et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer 2013, 13. [Google Scholar] [CrossRef]
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 promotes tumour incidence and growth. Nature 2006, 442, 461–465. [Google Scholar] [CrossRef]
- Muranski, P.; Boni, A.; Antony, P.A.; Cassard, L.; Irvine, K.R.; Kaiser, A.; Paulos, C.M.; Palmer, D.C.; Touloukian, C.E.; Ptak, K.; et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 2008, 112, 362–373. [Google Scholar] [CrossRef]
- Martin-Orozco, N.; Muranki, P.; Chung, Y.; Yang, X.O.; Yamazaki, T.; Lu, S.; Hwu, P.; Restifo, N.P.; Overwijk, W.W.; Dong, C. T helper 17 cells promote cytotoxic T-cell activation in tumor immunity. Immunity 2009, 31, 787–798. [Google Scholar] [CrossRef]
- Nunez, S.; Saez, J.J.; Fernandez, D.; Flores-Santibanez, F.; Alvarez, K.; Tejon, G.; Ruiz, P.; Maldonado, P.; Hidalgo, Y.; Manriquez, V.; et al. T helper type 17 cells contribute to anti-tumour immunity and promote the recruitment of T helper type 1 cells to the tumour. Immunology 2013, 139, 61–71. [Google Scholar] [CrossRef]
- Duran-Aniotz, C.; Segal, G.; Salazar, L.; Pereda, C.; Falcon, C.; Tempio, F.; Aguilera, R.; Gonzalez, R.; Perez, C.; Tittarelli, A.; et al. The immunological response and post-treatment survival of DC-vaccinated melanoma patients are associated with increased Th1/Th17 and reduced Th3 cytokine responses. Cancer Immunol. Immunother. 2013, 62, 761–772. [Google Scholar] [CrossRef]
- Lopez, M.N.; Pereda, C.; Segal, G.; Munoz, L.; Aguilera, R.; Gonzalez, F.E.; Escobar, A.; Ginesta, A.; Reyes, D.; Gonzalez, R.; et al. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor beta-expressing T-cells. J. Clin. Oncol. 2009, 27, 945–952. [Google Scholar] [CrossRef]
- Yu, Y.; Cho, H.I.; Wang, D.; Kaosaard, K.; Anasetti, C.; Celis, E.; Yu, X.Z. Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J. Immunol. 2013, 190, 1873–1881. [Google Scholar] [CrossRef]
- Garcia-Hernandez Mde, L.; Hamada, H.; Reome, J.B.; Misra, S.K.; Tighe, M.P.; Dutton, R.W. Adoptive transfer of tumor-specific Tc17 effector T-cells controls the growth of B16 melanoma in mice. J. Immunol. 2010, 184, 4215–4227. [Google Scholar] [CrossRef]
- Tajima, M.; Wakita, D.; Satoh, T.; Kitamura, H.; Nishimura, T. IL-17/IFN-gamma double producing CD8+ T (Tc17/IFN-gamma) cells: A novel cytotoxic T-cell subset converted from Tc17 cells by IL-12. Inte. Immunol. 2011, 23, 751–759. [Google Scholar] [CrossRef]
- Yeh, N.; Glosson, N.L.; Wang, N.; Guindon, L.; McKinley, C.; Hamada, H.; Li, Q.; Dutton, R.W.; Shrikant, P.; Zhou, B.; et al. Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype. J. Immunol. 2010, 185, 2089–2098. [Google Scholar] [CrossRef]
- Khan, A.; Fu, H.; Tan, L.A.; Harper, J.E.; Beutelspacher, S.C.; Larkin, D.F.; Lombardi, G.; McClure, M.O.; George, A.J. Dendritic cell modification as a route to inhibiting corneal graft rejection by the indirect pathway of allorecognition. Eur. J. Immunol. 2013, 43, 734–746. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef]
- Banchereau, J.; Ueno, H.; Dhodapker, M.; Connolly, J.; Finholt, J.P.; Klechevsky, E.; Blanck, J.P.; Johnston, D.A.; Palucka, A.K.; Fay, J.; et al. Immune and clinical outcomes in patients with stage IV melanoma vaccinated with peptide-pulsed dendritic cells derived from CD34+ progenitors and activated with type I interferon. J. Immunother. 2005, 28, 505–516. [Google Scholar] [CrossRef]
- Figdor, C.G.; de Vries, I.J.; Lesterhuis, W.J.; Melief, C.J. Dendritic cell immunotherapy: Mapping the way. Nat. Med. 2004, 10, 475–480. [Google Scholar] [CrossRef]
- Koski, G.K.; Koldovsky, U.; Xu, S.; Mick, R.; Sharma, A.; Fitzpatrick, E.; Weinstein, S.; Nisenbaum, H.; Levine, B.L.; Fox, K.; et al. A novel dendritic cell-based immunization approach for the induction of durable Th1-polarized anti-HER-2/neu responses in women with early breast cancer. J. Immunother. 2012, 35, 54–65. [Google Scholar] [CrossRef]
- Sharma, A.; Koldovsky, U.; Xu, S.; Mick, R.; Roses, R.; Fitzpatrick, E.; Weinstein, S.; Nisenbaum, H.; Levine, B.L.; Fox, P.; et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer 2012, 118, 4354–4362. [Google Scholar] [CrossRef]
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar]
- Hawiger, D.; Inaba, K.; Dorsett, Y.; Guo, M.; Mahnke, K.; Rivera, M.; Ravetch, J.V.; Steinman, R.M.; Nussenzweig, M.C. Dendritic cells induce peripheral T-cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001, 194, 769–779. [Google Scholar]
- Tarbell, K.V.; Yamazaki, S.; Olson, K.; Toy, P.; Steinman, R.M. CD25+ CD4+ T-cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 2004, 199, 1467–1477. [Google Scholar] [CrossRef]
- Ochando, J.C.; Homma, C.; Yang, Y.; Hidalgo, A.; Garin, A.; Tacke, F.; Angeli, V.; Li, Y.; Boros, P.; Ding, Y.; et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 2006, 7, 652–662. [Google Scholar] [CrossRef]
- Tokita, D.; Mazariegos, G.V.; Zahorchak, A.F.; Chien, N.; Abe, M.; Raimondi, G.; Thomson, A.W. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation 2008, 85, 369–377. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef]
- Thurner, B.; Roder, C.; Dieckmann, D.; Heuer, M.; Kruse, M.; Glaser, A.; Keikavoussi, P.; Kampgen, E.; Bender, A.; Schuler, G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods 1999, 223, 1–15. [Google Scholar] [CrossRef]
- Berger, T.G.; Feuerstein, B.; Strasser, E.; Hirsch, U.; Schreiner, D.; Schuler, G.; Schuler-Thurner, B. Large-scale generation of mature monocyte-derived dendritic cells for clinical application in cell factories. J. Immunol. Methods 2002, 268, 131–140. [Google Scholar] [CrossRef]
- Langenkamp, A.; Messi, M.; Lanzavecchia, A.; Sallusto, F. Kinetics of dendritic cell activation: Impact on priming of TH1, TH2 and nonpolarized T-cells. Nat. Immunol. 2000, 1, 311–316. [Google Scholar] [CrossRef]
- Dauer, M.; Obermaier, B.; Herten, J.; Haerle, C.; Pohl, K.; Rothenfusser, S.; Schnurr, M.; Endres, S.; Eigler, A. Mature dendritic cells derived from human monocytes within 48 hours: A novel strategy for dendritic cell differentiation from blood precursors. J. Immunol. 2003, 170, 4069–4076. [Google Scholar]
- Czerniecki, B.J.; Koski, G.K.; Koldovsky, U.; Xu, S.; Cohen, P.A.; Mick, R.; Nisenbaum, H.; Pasha, T.; Xu, M.; Fox, K.R.; et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007, 67, 1842–1852. [Google Scholar] [CrossRef]
- Jonuleit, H.; Kuhn, U.; Muller, G.; Steinbrink, K.; Paragnik, L.; Schmitt, E.; Knop, J.; Enk, A.H. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immun. 1997, 27, 3135–3142. [Google Scholar]
- Re, F.; Strominger, J.L. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 2001, 276, 37692–37699. [Google Scholar] [CrossRef]
- Macatonia, S.E.; Hosken, N.A.; Litton, M.; Vieira, P.; Hsieh, C.S.; Culpepper, J.A.; Wysocka, M.; Trinchieri, G.; Murphy, K.M.; O’Garra, A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T-cells. J. Immunol. 1995, 154, 5071–5079. [Google Scholar]
- Heufler, C.; Koch, F.; Stanzl, U.; Topar, G.; Wysocka, M.; Trinchieri, G.; Enk, A.; Steinman, R.M.; Romani, N.; Schuler, G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur. J. Immunol. 1996, 26, 659–668. [Google Scholar] [CrossRef]
- Hochrein, H.; Shortman, K.; Vremec, D.; Scott, B.; Hertzog, P.; O’Keeffe, M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J. Immunol. 2001, 166, 5448–5455. [Google Scholar]
- Shu, U.; Kiniwa, M.; Wu, C.Y.; Maliszewski, C.; Vezzio, N.; Hakimi, J.; Gately, M.; Delespesse, G. Activated T-cells induce interleukin-12 production by monocytes via CD40−CD40 ligand interaction. Eur. J. Immunol. 1995, 25, 1125–1128. [Google Scholar] [CrossRef]
- Cella, M.; Scheidegger, D.; Palmer-Lehmann, K.; Lane, P.; Lanzavecchia, A.; Alber, G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T-cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 1996, 184, 747–752. [Google Scholar] [CrossRef]
- Bianchi, R.; Grohmann, U.; Vacca, C.; Belladonna, M.L.; Fioretti, M.C.; Puccetti, P. Autocrine IL-12 is involved in dendritic cell modulation via CD40 ligation. J. Immunol. 1999, 163, 2517–2521. [Google Scholar]
- Wesa, A.; Galy, A. Increased production of pro-inflammatory cytokines and enhanced T-cell responses after activation of human dendritic cells with IL-1 and CD40 ligand. BMC Immunol. 2002, 3. [Google Scholar] [CrossRef]
- Koch, F.; Stanzi, U.; Jennewein, P.; Janke, K.; Heufler, C.; Kampgen, E.; Romani, N.; Schuler, G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 1996, 184, 741–746. [Google Scholar] [CrossRef]
- Vieira, P.L.; de Jong, E.C.; Wierenga, E.A.; Kapsenberg, M.L.; Kalinski, P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 2000, 164, 4507–4512. [Google Scholar]
- Jefford, M.; Schnurr, M.; Toy, T.; Masterman, K.A.; Shin, A.; Beecroft, T.; Tai, T.Y.; Shortman, K.; Shackleton, M.; David, I.D.; et al. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: Differential regulation of function by specific classes of physiologic stimuli. Blood 2003, 1753–1763. [Google Scholar]
- Snijders, A.; Kalinski, P.; Hilkens, C.M.; Kapsenberg, M.L. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 1998, 10, 1593–1598. [Google Scholar] [CrossRef]
- Verreck, F.A.; de Boer, T.; Langenberg, D.M.; Hoeve, M.A.; Kramer, M.; Vaisberg, E.; Kastelein, R.; Kolk, A.; de Waal-Malefyt, R.; Ottenhoff, T.H. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 4560–4565. [Google Scholar] [CrossRef]
- Roses, R.E.; Xu, S.; Xu, M.; Koldovsky, U.; Koski, G.; Czerniecki, B.J. Differential Production of IL-23 and IL-12 by Myeloid Dendritic Cells in response to TLR Agonists. J. Immunol. 2008, 181, 5120–5127. [Google Scholar]
- Smits, H.H.; van Beelen, A.J.; Hessle, C.; Westland, R.; de Jong, E.; Soeteman, E.; Wold, A.; Wierenga, E.A.; Kapsenberg, M.L. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur. J. Immunol. 2004, 34, 1371–1380. [Google Scholar] [CrossRef]
- Sallusto, F.; Schaerli, P.; Loetscher, P.; Schaniel, C.; Lenig, D.; Mackay, C.R.; Qin, S.; Lanzavecchia, A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998, 28, 2760–2769. [Google Scholar] [CrossRef]
- Yanagihara, S.; Komura, E.; Nagafune, J.; Watarai, H.; Yamaguchi, Y. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J. Immunol. 1998, 161, 3096–3102. [Google Scholar]
- Sanchez-Sanchez, N.; Riol-Blanco, L.; Rodriguez-Fernandez, J.L. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J. Immunol. 2006, 176, 5153–5159. [Google Scholar]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef]
- Paustian, C.; Taylor, P.; Johnson, T.; Xu, M.; Ramirez, N.; Rosenthal, K.S.; Shu, S.; Cohen, P.A.; Czerniecki, B.J.; Koski, G.K. Extracellular ATP and Toll-like receptor 2 agonists trigger in human monocytes an activation program that favors T helper 17. PLoS One 2013, 8, e54804. [Google Scholar] [CrossRef]
- Muthuswamy, R.; Mueller-Berghaus, J.; Haberkorn, U.; Reinhart, T.A.; Schadendorf, D.; Kalinski, P. PGE(2) transiently enhances DC expression of CCR7 but inhibits the ability of DCs to produce CCL19 and attract naive T-cells. Blood 2010, 116, 1454–1459. [Google Scholar] [CrossRef]
- Mailliard, R.B.; Wankowicz-Kalinska, A.; Cai, Q.; Wesa, A.; Hilkens, C.M.; Kapsenberg, M.L.; Kirkwood, J.M.; Storkus, W.J.; Kalinski, P. Alpha-type-1 polarized dendritic cells: A novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004, 64, 5934–5937. [Google Scholar] [CrossRef]
- Kalinski, P.; Vieira, P.L.; Schuitemaker, J.H.; de Jong, E.C.; Kapsenberg, M.L. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 2001, 97, 3466–3469. [Google Scholar] [CrossRef]
- Kalinski, P.; Hilkens, C.M.; Snijders, A.; Snijdewint, F.G.; Kapsenberg, M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 1997, 159, 28–35. [Google Scholar]
- Kalinski, P.; Hilkens, C.M.; Snijders, A.; Snijdewint, F.G.; Kapsenberg, M.L. Dendritic cells, obtained from peripheral blood precursors in the presence of PGE2, promote Th2 responses. Adv. Exp. Med. Biol. 1997, 417, 363–367. [Google Scholar] [CrossRef]
- Muthuswamy, R.; Urban, J.; Lee, J.J.; Reinhart, T.A.; Bartlett, D.; Kalinski, P. Ability of mature dendritic cells to interact with regulatory T-cells is imprinted during maturation. Cancer Res. 2008, 68, 5972–5978. [Google Scholar] [CrossRef]
- Baratelli, F.; Lin, Y.; Zhu, L.; Yang, S.C.; Heuze-Vourc’h, N.; Zeng, G.; Reckamp, K.; Dohadwala, M.; Sharma, S.; Dubinett, S.M. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T-cells. J. Immunol. 2005, 175, 1483–1490. [Google Scholar]
- Sharma, S.; Yang, S.C.; Zhu, L.; Reckamp, K.; Gardner, B.; Baratelli, F.; Huang, M.; Batra, R.K.; Dubinett, S.M. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005, 65, 5211–5220. [Google Scholar] [CrossRef]
- Chizzolini, C.; Chicheportiche, R.; Alvarez, M.; de Rham, C.; Roux-Lombard, P.; Ferrari-Lacraz, S.; Dayer, J.M. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood 2008, 112, 3696–3703. [Google Scholar] [CrossRef]
- Napolitani, G.; Acosta-Rodriquez, E.V.; Lanzavecchia, A.; Sallusto, F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-gamma production by memory CD4+ T-cells. Eur. J. Immun. 2009, 39, 1301–1312. [Google Scholar] [CrossRef]
- Dubsky, P.; Saito, H.; Leogier, M.; Dantin, C.; Connolly, J.E.; Banchereau, J.; Palucka, A.K. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T-cells to differentiate into CTL. Eur. J. Immunol. 2007, 37, 1678–1690. [Google Scholar] [CrossRef]
- Pulendran, B.; Dillon, S.; Joseph, C.; Curiel, T.; Banchereau, J.; Mohamadzadeh, M. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur. J. Immunol. 2004, 34, 66–73. [Google Scholar] [CrossRef]
- Mohamadzadeh, M.; Berard, F.; Essert, G.; Chalouni, C.; Pulendran, B.; Davoust, J.; Bridges, G.; Palucka, A.K.; Banchereau, J. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J. Exp. Med. 2001, 194, 1013–1020. [Google Scholar] [CrossRef]
- Harris, K.M. Monocytes differentiated with GM-CSF and IL-15 initiate Th17 and Th1 responses that are contact-dependent and mediated by IL-15. J. Leukocyte Biol. 2011, 90, 727–734. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Terhune, J.; Berk, E.; Czerniecki, B.J. Dendritic Cell-Induced Th1 and Th17 Cell Differentiation for Cancer Therapy. Vaccines 2013, 1, 527-549. https://doi.org/10.3390/vaccines1040527
Terhune J, Berk E, Czerniecki BJ. Dendritic Cell-Induced Th1 and Th17 Cell Differentiation for Cancer Therapy. Vaccines. 2013; 1(4):527-549. https://doi.org/10.3390/vaccines1040527
Chicago/Turabian StyleTerhune, Julia, Erik Berk, and Brian J. Czerniecki. 2013. "Dendritic Cell-Induced Th1 and Th17 Cell Differentiation for Cancer Therapy" Vaccines 1, no. 4: 527-549. https://doi.org/10.3390/vaccines1040527




