Chemokines as Cancer Vaccine Adjuvants
Abstract
:1. Introduction
| Chemokine standard name | Chemokine discovery name | Corresponding receptor | Functional category |
|---|---|---|---|
| CXCL1 | GROα/MGSA-α | CXCR2, CXCR1 | inflammatory and angiogenic |
| CXCL2 | GROβ/MGSA-β | CXCR2 | inflammatory and angiogenic |
| CXCL3 | GROγ/MGSA-γ | CXCR2 | inflammatory and angiogenic |
| CXCL4 | PF4 | CXCR3-B | angiostatic |
| CXCL5 | ENA-78 | CXCR2 | inflammatory and angiogenic |
| CXCL6 | GCP-2 | CXCR1, CXCR2 | inflammatory and angiogenic |
| CXCL7 | NAP-2 | CXCR1, CXCR2 | inflammatory and angiogenic |
| CXCL8 | IL-8 | CXCR1, CXCR2 | inflammatory and angiogenic |
| CXCL9 | MIG | CXCR3 | inflammatory and angiostatic |
| CXCL10 | IP-10 | CXCR3 | inflammatory and angiostatic |
| CXCL11 | I-TAC | CXCR3, CXCR7 | inflammatory and angiostatic |
| CXCL12 | SDF-1 | CXCR4, CXCR7 | homeostatic |
| CXCL13 | BCA-1 | CXCR5, CXCR3 | homeostatic |
| CXCL14 | BRAK/bolekine | unknown | Homeostatic |
| CXCL16 | SR-PSOX | CXCR6 | inflammatory |
| CXCL17 | DMC | unknown | homeostatic |
| XCL1 | lymphotactin/SCM-1α/ATAC | XCR1 | inflammatory and homeostatic |
| XCL2 | SCM-1β | XCR1 | inflammatory and homeostatic |
| CX3CL1 | Fractalkine | CX3CR1 | inflammatory, homeostatic and angiogenic |
| CCL1 | I-309 | CCR8 | inflammatory and angiogenic |
| CCL2 | MCP-1/MCAF/TDCF | CCR2 | inflammatory and angiogenic |
| CCL3 | MIP-1α/LD78α | CCR1, CCR5 | inflammatory |
| CCL3L1 | LD78β | CCR1, CCR5 | inflammatory |
| CCL4 | MIP-1β | CCR5 | inflammatory |
| CCL5 | RANTES | CCR1, CCR3, CCR5 | inflammatory |
| CCL7 | MCP-3 | CCR1, CCR2, CCR3 | inflammatory |
| CCL8 | MCP-2 | CCR3, CCR5 | inflammatory |
| CCL11 | Eotaxin-1 | CCR3 | inflammatory, homeostatic and angiogenic |
| CCL13 | MCP-4 | CCR2, CCR3 | inflammatory |
| CCL14 | HCC-1 | CCR1, CCR3, CCR5 | |
| CCL15 | HCC-2/Lkn-1/MIP-1δ | CCR1, CCR3 | |
| CCL16 | HCC-4/LEC/LCC-1 | CCR1, CCR2, CCR5 | |
| CCL17 | TARC | CCR4 | inflammatory and homeostatic |
| CCL18 | DC-CK1/PACRC/AMAC-1 | unknown | homeostatic |
| CCL19 | MIP-3β/ELC/exodus-3 | CCR7 | homeostatic |
| CCL20 | MIP-3α/LARC/exodus-1 | CCR6 | inflammatory and homeostatic |
| CCL21 | 6Ckine/SLC/exodus-2 | CCR7 | homeostatic |
| CCL22 | MDC/STCP-1 | CCR4 | inflammatory and homeostatic |
| CCL23 | MPIF-1/CKβ8/CKβ8-1 | CCR1 | |
| CCL24 | Eotaxin-2/MPIF-2 | CCR3 | homeostatic |
| CCL25 | TECK | CCR9 | homeostatic |
| CCL26 | Eotaxin-3 | CCR3 | inflammatory |
| CCL27 | CTACK/ILC | CCR10 | homeostatic |
| CCL28 | MEC | CCR3, CCR10 | homeostatic |
2. Chemokines Modify Effector Cell and APC Function
3. Chemokines as Adjuvants for Cancer Vaccines
| Vaccine Approach | Chemokine Approach | Cancer Type | Murine or Human | Reference |
|---|---|---|---|---|
| DC Vaccines | Use of CCL3 and CCL20 to improve DCs collection | Gastric Cancer | Murine | [24] |
| XCL1 + gp100 DC vaccine | Melanoma | Murine | [25] | |
| Pre-treatment of DCs with CCL3 | Melanoma | Murine | [26] | |
| Whole cell tumor lysate-pulsed DC vaccine transfected with CXCL10 pDNA | Glioma | Murine | [27] | |
| Insertion of CXCL10 gene into DCs | Cervical Cancer | Murine | [28] | |
| Whole cell tumor lysate-pulsed DC vaccine transfected with CCL21 | Prostate Cancer | Murine | [29] | |
| Conditioning DC vaccine site with irradiated CCL20-expressing tumor cells | Murine | [30] | ||
| DCs transfected with CCL21 gene | Hepatocellular Carcinoma | Murine | [31] | |
| DCs pulsed with whole tumor lysate and transfected with CXCL10 plasmid | Prostate | Murine | [32] | |
| Whole cell tumor lysate-pulsed DC vaccine combined with CCL5-containing vaccinia | Colon Cancer | Murine | [33] | |
| Intratumoral administration of gene-modified bone marrow DCs transduced with adenoviral vector expressing CCL21 | Lung Cancer | Murine | [34] | |
| βgal pDNA * + CCL19 pDNA | Fibrosarcoma Lymphoma | Murine | [35] | |
| Her2/neu pDNA + CCL19 pDNA | Breast | Murine | [36] | |
| TERT DNA vacccine primed with CCL21 | Breast | Murine | [37] | |
| DNA Vaccines | Ova pDNA + CCL5-Ig pDNA | Lymphoma | Murine | [38] |
| Her2/neu pDNA + CCL21 pDNA | Breast | Murine | [39] | |
| Ova pDNA + CX3CL1-Ig DNA | Lymphoma | Murine | [40] | |
| pCCL21&-HP (encodes for Her2/neu + p53)-Fc construct | Melanoma | Murine | [41] | |
| pCCL21-E7-Fc | Cervical Cancer | Murine | [42] | |
| pCCL21-3P-Fc | Melanoma | Murine | [43] | |
| CCL21 + TRP DNA vaccine | Melanoma | Murine | [44] | |
| CCL5pDNA + gp100 pDNA vaccine, with CCL5 + hgp100 viral vector boost | Melanoma | Murine | [45] | |
| CCL21 pDNA + hgp100 pDNA +/− IL2 | Melanoma | Murine | [46] | |
| Whole Cell/Lysate or Gene Modified Cancer Cells | CCL21-expressing tumor cells | Melanoma | Murine | [47] |
| CCL3+ IL2 or CCL3+ GMCSF | Leukemia/lymphoma | Murine | [48] | |
| B16F0 transfected with pCCL21-3p-Fc | Melanoma | Murine | [49] | |
| GMCSF-producing WEHI3B with recombinant CCL17 or CCL5 | Murine Myelomonocytic Leukemia | Murine | [50] | |
| Glioma cell vaccine expressing CCL3 and GM-CSF | Glioma | Murine | [51] | |
| IL2 + GMCSF expressing Meth A and HM-1 tumor cells co-transfected with CCL21, CCL19 and CXCL12 | Fibrosarcoma and Ovarian Cancer | Murine | [52] | |
| TAA-Chemokines | Fusion of CCL7, CCL20, CXCL10 to TAA | B Cell Lymphoma | Murine | [53,54] |
| Type of vaccine | Trial description | Phase | Cancer Type | Status | Published? |
|---|---|---|---|---|---|
| DC | Intradermal injection of adenovirus-CCL21 transduced class I peptide-pulsed DCs [55] | Phase I | Melanoma | closed | no |
| Intratumoral autologous DC-adenovirus CCL21 vaccine [56,57] | Phase I | Stage IIIB-IV or recurrent Non-Small Cell Lung Cancer | open | no | |
| Genetically-modified Cancer Cells | Combination immunotherapy of GM.CD40L * vaccine with CCL21 [58] | Phase I | Lung Cancer | open | no |
| Gene-modified tumor cells for relapsed/refractory disease (CYCHE) [59] | Phase I | Neuroblastoma | completed | no | |
| A phase I/II study of immunization with XCL1 and IL-2 gene modified tumor vaccine (CHESAT) [60] | Phase I/II | Neuroblastoma | open | no | |
| Allogeneic tumor cells for relapsed/refractory disease (CYCHEALL) [61] | Phase I | Neuroblastoma | open | no | |
| TDNA vaccines | Phase I study for asymptomatic Phase disease with DNA vaccines encoding antigen-chemokine fusion [62] | Phase I | Asymptomatic Phase Lympho-plasmacytic Lymphoma | Not yet open | no |
3.1. Use of Chemokines to Enhance DC Vaccines: A Field Moving towards Phase I-II Clinical Trials
3.2. Chemokine Adjuvants to DNA Vaccines
3.3. Transforming Non-Immunogenic TAAs into Cancer Vaccines by Fusion with Chemokines
3.4. Whole Cell/Lysate Cancer Vaccines and Gene-Modified Tumor Vaccines: From Bench to Clinical Trials
4. Exceptions to the Positive Effect of Chemokine Adjuvants in Tumor Vaccines
5. Future Perspective
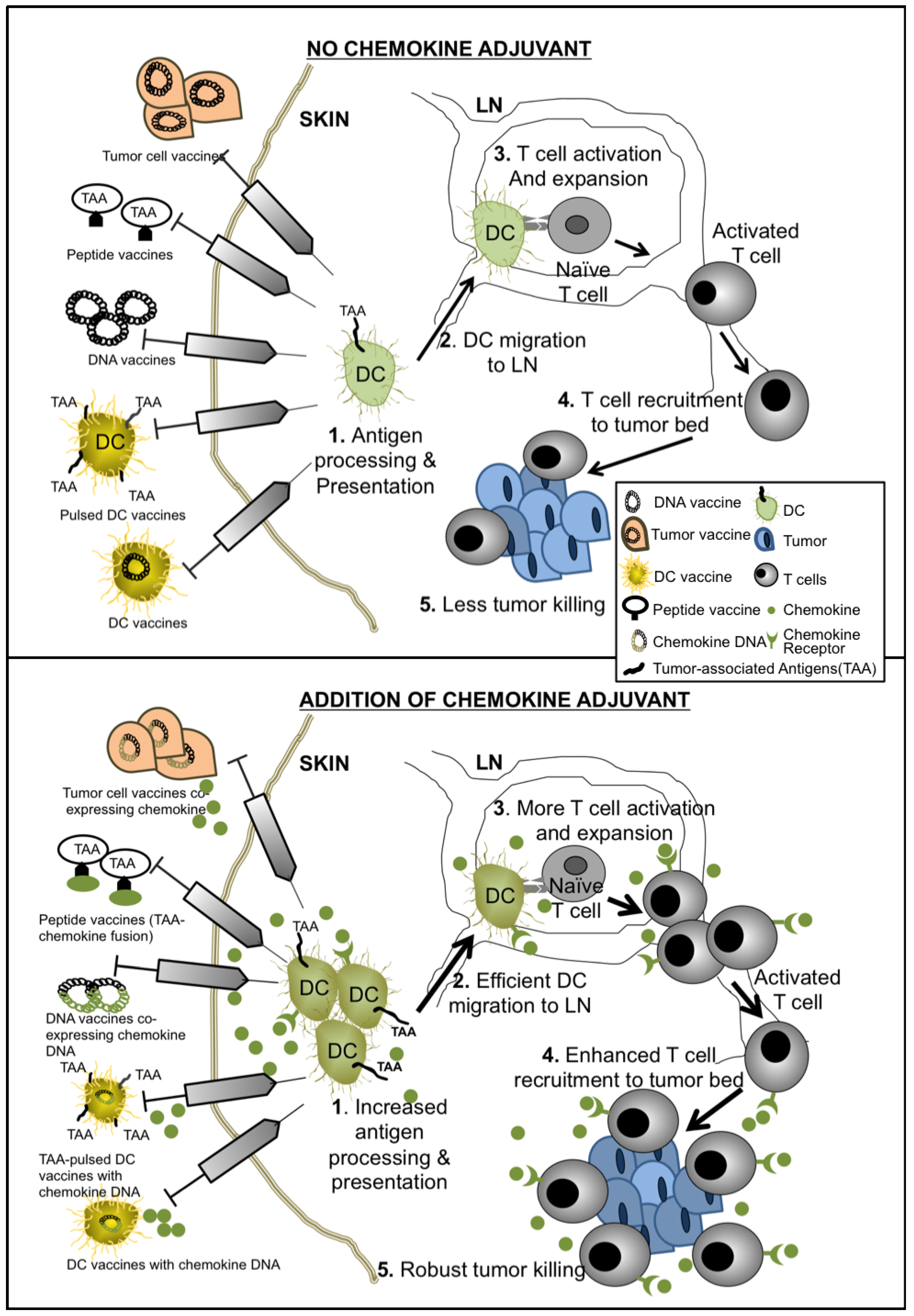
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dell’Agnola, C.; Biragyn, A. Clinical utilization of chemokines to combat cancer: The double-edged sword. Expert Rev. Vaccines 2007, 6, 267–283. [Google Scholar] [CrossRef]
- Luther, S.A.; Cyster, J.G. Chemokines as regulators of T cell differentiation. Nat. Immunol. 2001, 2, 102–107. [Google Scholar] [CrossRef]
- Castellino, F.; Huang, A.Y.; Altan-Bonnet, G.; Stoll, S.; Scheinecker, C.; Germain, R.N. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 2006, 440, 890–895. [Google Scholar] [CrossRef]
- Lechner, M.G.; Russell, S.M.; Bass, R.S.; Epstein, A.L. Chemokines, costimulatory molecules and fusion proteins for the immunotherapy of solid tumors. Immunotherapy 2011, 3, 1317–1340. [Google Scholar] [CrossRef]
- Franciszkiewicz, K.; Boissonnas, A.; Boutet, M.; Combadière, C.; Mami-Chouaib, F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012, 72, 6325–6332. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef]
- Belperio, J.A.; Keane, M.P.; Arenberg, D.A.; Addison, C.L.; Ehlert, J.E.; Burdick, M.D.; Strieter, M. CXC chemokines in angiogenesis. J. Leukoc Biol. 2000, 68, 1–8. [Google Scholar]
- Coscia, M.; Biragyn, A. Cancer immunotherapy with chemoattractant peptides. Semin. Cancer Biol. 2004, 14, 209–218. [Google Scholar] [CrossRef]
- McColl, S.R. Chemokines and dendritic cells: A crucial alliance. Immunol. Cell Biol. 2002, 80, 489–496. [Google Scholar] [CrossRef]
- Dieu, M.C.; Vanbervliet, B.; Vicari, A.; Bridon, J.M.; Oldham, E.; Aït-Yahia, S.; Brière, F.; Zlotnik, A.; Lebecque, S.; Caux, C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998, 188, 373–386. [Google Scholar] [CrossRef]
- Sallusto, F.; Palermo, B.; Lenig, D.; Miettinen, M.; Matikainen, S.; Julkunen, I.; Forster, R.; Burgstahler, R.; Lipp, M.; Lanzavecchia, A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999, 29, 1617–1625. [Google Scholar] [CrossRef]
- Smith, C.M.; Wilson, N.S.; Waithman, J.; Villadangos, J.A.; Carbone, F.R.; Heath, W.R.; Belz, G.T. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nat. Immunol. 2004, 5, 1143–1148. [Google Scholar] [CrossRef]
- Mandl, J.N.; Liou, R.; Klauschen, F.; Vrisekoop, N.; Monteiro, J.P.; Yates, A.J.; Huang, A.Y.; Germain, R.N. Quantification of lymph node transit times reveals differences in antigen surveillance strategies of naive CD4+ and CD8+ T cells. Proc. Natl. Acad. Sci. USA 2012, 109, 18036–18041. [Google Scholar] [CrossRef]
- Hugues, S.; Scholer, A.; Boissonnas, A.; Nussbaum, A.; Combadière, C.; Amigorena, S.; Fetler, L. Dynamic imaging of chemokine-dependent CD8+ T cell help for CD8+ T cell responses. Nat. Immunol. 2007, 8, 921–930. [Google Scholar]
- Kaiser, A.; Donnadieu, E.; Abastado, J.P.; Trautmann, A.; Nardin, A. CC chemokine ligand 19 secreted by mature dendritic cells increases naive T cell scanning behavior and their response to rare cognate antigen. J. Immunol. 2005, 175, 2349–2356. [Google Scholar]
- Molon, B.; Gri, G.; Bettella, M.; Gómez-Moutón, C.; Lanzavecchia, A.; Martínez-A, C.; Mañes, S.; Viola, A. T cell costimulation by chemokine receptors. Nat. Immunol. 2005, 6, 465–471. [Google Scholar]
- Moser, B.; Loetscher, P. Lymphocyte traffic control by chemokines. Nat. Immunol. 2001, 2, 123–128. [Google Scholar] [CrossRef]
- Fujita, Y.; Taguchi, H. Overview and outlook of Toll-like receptor ligand-antigen conjugate vaccines. Ther. Deliv. 2012, 3, 749–760. [Google Scholar]
- Zom, G.G.; Khan, S.; Filippov, D.V.; Ossendorp, F. TLR ligand-peptide conjugate vaccines: Toward clinical application. Adv. Immunol. 2012, 114, 177–201. [Google Scholar] [CrossRef]
- Duthie, M.S.; Windish, H.P.; Fox, C.B.; Reed, S.G. Use of defined TLR ligands as adjuvants within human vaccines. Immunol. Rev. 2011, 239, 178–196. [Google Scholar] [CrossRef]
- Ribas, A.; Butterfield, L.H.; Glaspy, J.A.; Economou, J.S. Current developments in cancer vaccines and cellular immunotherapy. J. Clin. Oncol. 2003, 21, 2415–2432. [Google Scholar] [CrossRef]
- Rosenberg, S.A. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity 1999, 10, 281–287. [Google Scholar] [CrossRef]
- Schlom, J. Therapeutic cancer vaccines: Current status and moving forward. J. Natl. Cancer Inst. 2012, 104, 599–613. [Google Scholar] [CrossRef]
- He, S.; Wang, L.; Wu, Y.; Li, D.; Zhang, Y. CCL3 and CCL20-recruited dendritic cells modified by melanoma antigen gene-1 induce anti-tumor immunity against gastric cancer ex vivo and in vivo. J. Exp. Clin. Cancer Res. 2010. [Google Scholar] [CrossRef]
- Xia, D.J.; Zhang, W.P.; Zheng, S.; Wang, J.; Pan, J.P.; Wang, Q.; Zhang, L.H.; Hamada, H.; Cao, X. Lymphotactin cotransfection enhances the therapeutic efficacy of dendritic cells genetically modified with melanoma antigen gp100. Gene Ther. 2002, 9, 592–601. [Google Scholar] [CrossRef]
- Cao, Q.; Jin, Y.; Jin, M.; He, S.; Gu, Q.; Qiu, Y.; Ge, H.; Yoneyama, H.; Zhang, Y. Therapeutic effect of MIP-1alpha-recruited dendritic cells on preestablished solid and metastatic tumors. Cancer Lett. 2010, 295, 17–26. [Google Scholar] [CrossRef]
- Jiang, X.B.; Lu, X.L.; Hu, P.; Liu, R.E. Improved therapeutic efficacy using vaccination with glioma lysate-pulsed dendritic cells combined with IP-10 in murine glioma. Vaccine 2009, 27, 6210–6216. [Google Scholar] [CrossRef]
- Kang, T.H.; Bae, H.C.; Kim, S.H.; Seo, S.H.; Son, S.W.; Choi, E.Y.; Seong, S.Y.; Kim, T.W. Modification of dendritic cells with interferon-gamma-inducible protein-10 gene to enhance vaccine potency. J. Gene Med. 2009, 11, 889–898. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Q.; Liang, C.M.; Xia, S.J.; Zhong, C.P.; Wang, D.W. Antitumor immunity by a dendritic cell vaccine encoding secondary lymphoid chemokine and tumor lysate on murine prostate cancer. Asian J. Androl. 2008, 10, 883–889. [Google Scholar] [CrossRef]
- Shih, N.Y.; Yang, H.Y.; Cheng, H.T.; Hung, Y.M.; Yao, Y.C.; Zhu, Y.H.; Wu, Y.C.; Liu, K.J. Conditioning vaccination site with irradiated MIP-3alpha-transfected tumor cells enhances efficacy of dendritic cell-based cancer vaccine. J. Immunother. 2009, 32, 363–369. [Google Scholar] [CrossRef]
- Liang, C.M.; Ye, S.L.; Zhong, C.P.; Zheng, N.; Bian, W.; Sun, R.X.; Chen, J.; Li, R.L.; Zhou, S.; Liu, Y.K. More than chemotaxis: A new anti-tumor DC vaccine modified by rAAV2-SLC. Mol. Immunol. 2007, 44, 3797–804. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Zhao, Q.L.; Wei, X.B.; Zhang, X.H.; Wu, C.Q.; Zhong, C.P. Murine dendritic cells modified with CXCL10 gene and tumour cell lysate mediate potent antitumour immune responses in mice. Scand. J. Immunol. 2007, 65, 8–13. [Google Scholar] [CrossRef]
- Li, J.; O’Malley, M.; Urban, J.; Sampath, P.; Guo, Z.S.; Kalinski, P.; Thorne, S.H.; Bartlett, D.L. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol. Ther. 2011, 19, 650–657. [Google Scholar] [CrossRef]
- Yang, S.C.; Hillinger, S.; Riedl, K.; Zhang, L.; Zhu, L.; Huang, M.; Atianzar, K.; Kuo, B.Y.; Gardner, B.; Batra, R.K.; et al. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin. Cancer Res. 2004, 10, 2891–2901. [Google Scholar] [CrossRef]
- Westermann, J.; Nguyen-Hoai, T.; Baldenhofer, G.; Höpken, U.E.; Lipp, M.; Dörken, B.; Pezzutto, A. CCL19 (ELC) as an adjuvant for DNA vaccination: Induction of a TH1-type T-cell response and enhancement of antitumor immunity. Cancer Gene Ther. 2007, 14, 523–532. [Google Scholar]
- Nguyen-Hoai, T.; Baldenhofer, G.; Ahmed, M.S.; Pham-Duc, M.; Gries, M.; Lipp, M.; Dörken, B.; Pezzutto, A.; Westermann, J. CCL19 (ELC) improves TH1-polarized immune responses and protective immunity in a murine Her2/neu DNA vaccination model. J. Gene Med. 2012, 14, 128–137. [Google Scholar] [CrossRef]
- Yamano, T.; Kaneda, Y.; Hiramatsu, S.H.; Huang, S.; Tran, A.N.; Giuliano, A.E.; Hoon, D.S. Immunity against breast cancer by TERT DNA vaccine primed with chemokine CCL21. Cancer Gene Ther. 2007, 14, 451–459. [Google Scholar]
- Dorgham, K.; Abadie, V.; Iga, M.; Hartley, O.; Gorochov, G.; Combadière, B. Engineered CCR5 superagonist chemokine as adjuvant in anti-tumor DNA vaccination. Vaccine 2008, 26, 3252–3260. [Google Scholar] [CrossRef]
- Nguyen-Hoai, T.; Baldenhofer, G.; Sayed Ahmed, M.S.; Pham-Duc, M.; Vu, M.D.; Lipp, M.; Dörken, B.; Pezzutto, A.; Westermann, J. CCL21 (SLC) improves tumor protection by a DNA vaccine in a Her2/neu mouse tumor model. Cancer Gene Ther. 2012, 19, 69–76. [Google Scholar] [CrossRef]
- Iga, M.; Boissonnas, A.; Mahé, B.; Bonduelle, O.; Combadière, C.; Combadière, B. Single CX3CL1-Ig DNA administration enhances T cell priming in vivo. Vaccine 2007, 25, 4554–4563. [Google Scholar] [CrossRef]
- Sun, W.; Qian, H.; Zhang, X.; Zhou, C.; Liang, X.; Wang, D.; Fu, M.; Zhang, S.; Lin, C. Induction of protective and therapeutic antitumour immunity using a novel tumour-associated antigen-specific DNA vaccine. Immunol. Cell Biol. 2006, 84, 440–447. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, C.; Wang, D.; Ma, W.; Lin, C.; Wang, Y.; Zhang, Y.; Zhang, S. Enhancement of DNA vaccine potency by sandwiching antigen-coding gene between secondary lymphoid tissue chemokine (SLC) and IgG Fc fragment genes. Cancer Biol. Ther. 2006, 5, 427–434. [Google Scholar] [CrossRef]
- Qin, H.; Zhou, C.; Wang, D.; Ma, W.; Liang, X.; Lin, C.; Zhang, Y.; Zhang, S. Enhancement of antitumour immunity by a novel chemotactic antigen DNA vaccine encoding chemokines and multiepitopes of prostate-tumour-associated antigens. Immunology 2006, 117, 419–430. [Google Scholar] [CrossRef]
- Yamano, T.; Kaneda, Y.; Huang, S.; Hiramatsu, S.H.; Hoon, D.S. Enhancement of immunity by a DNA melanoma vaccine against TRP2 with CCL21 as an adjuvant. Mol. Ther. 2006, 13, 194–202. [Google Scholar] [CrossRef]
- Aravindaram, K.; Yu, H.H.; Lan, C.W.; Wang, P.H.; Chen, Y.H.; Chen, H.M.; Yagita, H.; Yang, N.S. Transgenic expression of human gp100 and RANTES at specific time points for suppression of melanoma. Gene Ther. 2009, 16, 1329–1339. [Google Scholar] [CrossRef]
- Elzaouk, L.; Pavlovic, J.; Moelling, K. Analysis of antitumor activity elicited by vaccination with combinations of interleukin-12 DNA with gp100 DNA or the chemokine CCL21 in vivo. Hum. Gene Ther. 2006, 17, 859–870. [Google Scholar] [CrossRef]
- Novak, L.; Igoucheva, O.; Cho, S.; Alexeev, V. Characterization of the CCL21-mediated melanoma-specific immune responses and in situ melanoma eradication. Mol. Cancer Ther. 2007, 6, 1755–1764. [Google Scholar] [CrossRef]
- Zibert, A.; Balzer, S.; Souquet, M.; Quang, T.H.; Paris-Scholz, C.; Roskrow, M.; Dilloo, D. CCL3/MIP-1alpha is a potent immunostimulator when coexpressed with interleukin-2 or granulocyte-macrophage colony-stimulating factor in a leukemia/lymphoma vaccine. Hum. Gene Ther. 2004, 15, 21–34. [Google Scholar] [CrossRef]
- Li, N.; Qin, H.; Li, X.; Zhou, C.; Wang, D.; Ma, W.; Lin, C.; Zhang, Y.; Wang, S.; Zhang, S. Potent systemic antitumor immunity induced by vaccination with chemotactic-prostate tumor associated antigen gene-modified tumor cell and blockade of B7-H1. J. Clin. Immunol. 2007, 27, 117–130. [Google Scholar] [CrossRef]
- Inoue, H.; Iga, M.; Xin, M.; Asahi, S.; Nakamura, T.; Kurita, R.; Nakayama, M.; Nakazaki, Y.; Takayama, K.; Nakanishi, Y.; et al. TARC and RANTES enhance antitumor immunity induced by the GM-CSF-transduced tumor vaccine in a mouse tumor model. Cancer Immunol. Immunother. 2008, 57, 1399–1411. [Google Scholar] [CrossRef]
- Herrlinger, U.; Aulwurm, S.; Strik, H.; Weit, S.; Naumann, U.; Weller, M. MIP-1alpha antagonizes the effect of a GM-CSF-enhanced subcutaneous vaccine in a mouse glioma model. J. Neurooncol. 2004, 66, 147–154. [Google Scholar] [CrossRef]
- Nomura, T.; Hasegawa, H.; Kohno, M.; Sasaki, M.; Fujita, S. Enhancement of anti-tumor immunity by tumor cells transfected with the secondary lymphoid tissue chemokine EBI-1-ligand chemokine and stromal cell-derived factor-1alpha chemokine genes. Int. J. Cancer 2001, 91, 597–606. [Google Scholar] [CrossRef]
- Biragyn, A.; Schiavo, R.; Olkhanud, P.; Sumitomo, K.; King, A.; McCain, M.; Indig, F.E.; Almanzar, G.; Baatar, D. Tumor-associated embryonic antigen-expressing vaccines that target CCR6 elicit potent CD8+ T cell-mediated protective and therapeutic antitumor immunity. J. Immunol. 2007, 179, 1381–1388. [Google Scholar]
- Biragyn, A.; Tani, K.; Grimm, M.C.; Weeks, S.; Kwak, L.W. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat. Biotechnol. 1999, 17, 253–258. [Google Scholar] [CrossRef]
- Weber, J. Adenovirus CCL-21 Transduced MART-1/gp100/Tyrosinase/NY-ESO-1 Peptide-Pulsed Dendritic Cells Matured. Available online: http://clinicaltrials.gov/ct2/show/NCT007986292012 (accessed on 20 July 2013).
- Lee, J. Vaccine Therapy in Treating Patients With Stage IIIB, Stage IV, or Recurrent Non-Small Cell Lung Cancer. Available online: http://clinicaltrials.gov/ct2/show/NCT006010942013 (accessed on 20 July 2013).
- Dubinett, S. A Vaccine Trial for Patients With Stage IIIB, IV, or Recurrent Non-Small Cell Lung Cancer (VTNSCLC). Available online: http://clinicaltrials.gov/ct2/show/NCT015742222013 (accessed on 20 July 2013).
- Gray, J. Combination Immunotherapy of GM.CD40L Vaccine With CCL21 in Lung Cancer. Available online: http://clinicaltrials.gov/ct2/show/NCT014331722013 (accessed on 20 July 2013).
- Brenner, M. Using Gene Modified Neuroblastoma Cells for the Treatment of Relapsed/Refractory Neuroblastoma (CYCHE). Available online: http://clinicaltrials.gov/ct2/show/NCT000628552012 (accessed on 20 July 2013).
- Louis, C. A Phase I/II Study Of Immunization With Lymphotactin And Interleukin 2 Gene Modified Neuroblastoma Tumor Cells (CHESAT). Available online: http://clinicaltrials.gov/ct2/show/NCT007032222013 (accessed on 20 July 2013).
- Brenner, M. Allogeneic Neuroblastoma Cells for Relapsed/ Refractory Neuroblastoma, CYCHEALL. Available online: http://clinicaltrials.gov/ct2/show/NCT017134392012 (accessed on 20 July 2013).
- Thomas, S. Immunotherapy for Asymptomatic Phase Lymphoplasmacytic Lymphoma. Available online: http://clinicaltrials.gov/ct2/show/NCT012098712013 (accessed on 20 July 2013).
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Frankenberger, B.; Schendel, D.J. Third generation dendritic cell vaccines for tumor immunotherapy. Eur. J. Cell Biol. 2012, 91, 53–58. [Google Scholar] [CrossRef]
- Nishimura, F.; Dusak, J.E.; Eguchi, J.; Zhu, X.; Gambotto, A.; Storkus, W.J.; Okada, H. Adoptive transfer of type 1 CTL mediates effective anti-central nervous system tumor response: Critical roles of IFN-inducible protein-10. Cancer Res. 2006, 66, 4478–4487. [Google Scholar] [CrossRef]
- Serra, H.M.; Baena-Cagnani, C.E.; Eberhard, Y. Is secondary lymphoid-organ chemokine (SLC/CCL21) much more than a constitutive chemokine? Allergy 2004, 59, 1219–1223. [Google Scholar] [CrossRef]
- Fushimi, T.; Kojima, A.; Moore, M.A.; Crystal, R.G. Macrophage inflammatory protein 3alpha transgene attracts dendritic cells to established murine tumors and suppresses tumor growth. J. Clin. Invest. 2000, 105, 1383–1393. [Google Scholar] [CrossRef]
- Hedrick, J.A.; Zlotnik, A. Lymphotactin. Clin. Immunol. Immunopathol. 1998, 87, 218–222. [Google Scholar] [CrossRef]
- Wong, M.M.; Fish, E.N. Chemokines: Attractive mediators of the immune response. Semin. Immunol. 2003, 15, 5–14. [Google Scholar] [CrossRef]
- Marsland, B.J.; Bättig, P.; Bauer, M.; Ruedl, C.; Lässing, U.; Beerli, R.R.; Dietmeier, K.; Ivanova, L.; Pfister, T.; Vogt, L.; et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity 2005, 22, 493–505. [Google Scholar] [CrossRef]
- Kumamoto, T.; Huang, E.K.; Paek, H.J.; Morita, A.; Matsue, H.; Valentini, R.F.; Takashima, A. Induction of tumor-specific protective immunity by in situ Langerhans cell vaccine. Nat. Biotechnol. 2002, 20, 64–69. [Google Scholar] [CrossRef]
- Terando, A.; Roessler, B.; Mulé, J.J. Chemokine gene modification of human dendritic cell-based tumor vaccines using a recombinant adenoviral vector. Cancer Gene Ther. 2004, 11, 165–173. [Google Scholar] [CrossRef]
- Senovilla, L.; Vacchelli, E.; Garcia, P.; Eggermont, A.; Fridman, W.H.; Galon, J.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: DNA vaccines for cancer therapy. Oncoimmunology 2013, 2, e23803. [Google Scholar] [CrossRef]
- Pavlenko, M.; Leder, C.; Pisa, P. Plasmid DNA vaccines against cancer: Cytotoxic T-lymphocyte induction against tumor antigens. Expert Rev. Vaccines 2005, 4, 315–327. [Google Scholar] [CrossRef]
- Fioretti, D.; Iurescia, S.; Fazio, V.M.; Rinaldi, M. DNA vaccines: Developing new strategies against cancer. J. Biomed. Biotechnol. 2010. [Google Scholar] [CrossRef]
- Mohit, E.; Rafati, S. Chemokine-based immunotherapy: Delivery systems and combination therapies. Immunotherapy 2012, 4, 807–840. [Google Scholar] [CrossRef]
- Biragyn, A.; Surenhu, M.; Yang, D.; Ruffini, P.A.; Haines, B.A.; Klyushnenkova, E.; Oppenheim, J.J.; Kwak, L.W. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J. Immunol. 2001, 167, 6644–6653. [Google Scholar]
- Ruffini, P.A.; Biragyn, A.; Coscia, M.; Harvey, L.K.; Cha, S.C.; Bogen, B.; Kwak, L.W. Genetic fusions with viral chemokines target delivery of nonimmunogenic antigen to trigger antitumor immunity independent of chemotaxis. J. Leukoc. Biol. 2004, 76, 77–85. [Google Scholar] [CrossRef]
- Zaliauskiene, L.; Kang, S.; Sparks, K.; Zinn, K.R.; Schwiebert, L.M.; Weaver, C.T.; Collawn, J.F. Enhancement of MHC class II-restricted responses by receptor-mediated uptake of peptide antigens. J. Immunol. 2002, 169, 2337–2345. [Google Scholar]
- Mahnke, K.; Guo, M.; Lee, S.; Sepulveda, H.; Swain, S.L.; Nussenzweig, M.; Steinman, R.M. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J. Cell Biol. 2000, 151, 673–684. [Google Scholar] [CrossRef]
- Schiavo, R.; Baatar, D.; Olkhanud, P.; Indig, F.E.; Restifo, N.; Taub, D.; Biragyn, A. Chemokine receptor targeting efficiently directs antigens to MHC class I pathways and elicits antigen-specific CD8+ T-cell responses. Blood 2006, 107, 4597–4605. [Google Scholar] [CrossRef]
- Kryczek, I.; Wei, S.; Keller, E.; Liu, R.; Zou, W. Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis. Am. J. Physiol. Cell Physiol. 2007, 292, C987–C995. [Google Scholar] [CrossRef]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauack, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef]
- Scholler, J.; Brady, T.L.; Binder-Scholl, G.; Hwang, W.T.; Plesa, G.; Hege, K.M.; Vogel, A.N.; kalos, M.; Riley, J.L.; Riley, J.L.; et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012. [Google Scholar] [CrossRef]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Lipson, E.J.; Sharfman, W.H.; Drake, C.G.; Wollner, I.; Taube, J.M.; Anders, R.A.; Xu, H.; Yao, S.; Pons, A.; Chen, L.; et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin. Cancer Res. 2013, 19, 462–468. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bobanga, I.D.; Petrosiute, A.; Huang, A.Y. Chemokines as Cancer Vaccine Adjuvants. Vaccines 2013, 1, 444-462. https://doi.org/10.3390/vaccines1040444
Bobanga ID, Petrosiute A, Huang AY. Chemokines as Cancer Vaccine Adjuvants. Vaccines. 2013; 1(4):444-462. https://doi.org/10.3390/vaccines1040444
Chicago/Turabian StyleBobanga, Iuliana D., Agne Petrosiute, and Alex Y. Huang. 2013. "Chemokines as Cancer Vaccine Adjuvants" Vaccines 1, no. 4: 444-462. https://doi.org/10.3390/vaccines1040444
APA StyleBobanga, I. D., Petrosiute, A., & Huang, A. Y. (2013). Chemokines as Cancer Vaccine Adjuvants. Vaccines, 1(4), 444-462. https://doi.org/10.3390/vaccines1040444




