Deoxynivanelol and Fumonisin, Alone or in Combination, Induce Changes on Intestinal Junction Complexes and in E-Cadherin Expression
Abstract
:1. Introduction
2. Results
2.1. Histological Analysis
2.2. Expression of E-Cadherin
2.3. Ultrastructural Analysis
 ) or culture medium with fumonisin B1 (FB1) (
) or culture medium with fumonisin B1 (FB1) (  ), deoxynivalenol (DON) (
), deoxynivalenol (DON) (  ) or FB1 + DON (■). (A) Control explants. Edema of the lamina propria and mild villi atrophy (arrow); (B) FB1-exposed explant. Moderate fusion (arrow) and villous atrophy; (C) DON-exposed explant. Severe loss of apical enterocytes, fusion and atrophy (arrow); (D) FB1 + DON-exposed explant. Lysis of intestinal epithelium, villi atrophy, fusion (arrow) and cell debris. HE. Bar 100 µm; (E) Control explant showing a strong and homogeneous E-cadherin expression. Bar 20 µm; (F) DON-exposed explant showing reduced expression of E-cadherin. Bar 20 µm; (G) Tissue scores of pig intestinal explants exposed to FB1, DON and both mycotoxins; (H) Villi height in pig intestinal explants treated with FB1, DON and FB1 + DON; (I) Number of goblet cells per villus of pig intestinal explants treated with FB1, DON and FB1 + DON. Values are means with their standard deviation of the mean represented by vertical bars (n 5 animals). Mean values with unlike letters were significantly different (p ≤ 0.05). AU = Arbitrary Units.
) or FB1 + DON (■). (A) Control explants. Edema of the lamina propria and mild villi atrophy (arrow); (B) FB1-exposed explant. Moderate fusion (arrow) and villous atrophy; (C) DON-exposed explant. Severe loss of apical enterocytes, fusion and atrophy (arrow); (D) FB1 + DON-exposed explant. Lysis of intestinal epithelium, villi atrophy, fusion (arrow) and cell debris. HE. Bar 100 µm; (E) Control explant showing a strong and homogeneous E-cadherin expression. Bar 20 µm; (F) DON-exposed explant showing reduced expression of E-cadherin. Bar 20 µm; (G) Tissue scores of pig intestinal explants exposed to FB1, DON and both mycotoxins; (H) Villi height in pig intestinal explants treated with FB1, DON and FB1 + DON; (I) Number of goblet cells per villus of pig intestinal explants treated with FB1, DON and FB1 + DON. Values are means with their standard deviation of the mean represented by vertical bars (n 5 animals). Mean values with unlike letters were significantly different (p ≤ 0.05). AU = Arbitrary Units.
 ) or culture medium with fumonisin B1 (FB1) (
) or culture medium with fumonisin B1 (FB1) (  ), deoxynivalenol (DON) (
), deoxynivalenol (DON) (  ) or FB1 + DON (■). (A) Control explants. Edema of the lamina propria and mild villi atrophy (arrow); (B) FB1-exposed explant. Moderate fusion (arrow) and villous atrophy; (C) DON-exposed explant. Severe loss of apical enterocytes, fusion and atrophy (arrow); (D) FB1 + DON-exposed explant. Lysis of intestinal epithelium, villi atrophy, fusion (arrow) and cell debris. HE. Bar 100 µm; (E) Control explant showing a strong and homogeneous E-cadherin expression. Bar 20 µm; (F) DON-exposed explant showing reduced expression of E-cadherin. Bar 20 µm; (G) Tissue scores of pig intestinal explants exposed to FB1, DON and both mycotoxins; (H) Villi height in pig intestinal explants treated with FB1, DON and FB1 + DON; (I) Number of goblet cells per villus of pig intestinal explants treated with FB1, DON and FB1 + DON. Values are means with their standard deviation of the mean represented by vertical bars (n 5 animals). Mean values with unlike letters were significantly different (p ≤ 0.05). AU = Arbitrary Units.
) or FB1 + DON (■). (A) Control explants. Edema of the lamina propria and mild villi atrophy (arrow); (B) FB1-exposed explant. Moderate fusion (arrow) and villous atrophy; (C) DON-exposed explant. Severe loss of apical enterocytes, fusion and atrophy (arrow); (D) FB1 + DON-exposed explant. Lysis of intestinal epithelium, villi atrophy, fusion (arrow) and cell debris. HE. Bar 100 µm; (E) Control explant showing a strong and homogeneous E-cadherin expression. Bar 20 µm; (F) DON-exposed explant showing reduced expression of E-cadherin. Bar 20 µm; (G) Tissue scores of pig intestinal explants exposed to FB1, DON and both mycotoxins; (H) Villi height in pig intestinal explants treated with FB1, DON and FB1 + DON; (I) Number of goblet cells per villus of pig intestinal explants treated with FB1, DON and FB1 + DON. Values are means with their standard deviation of the mean represented by vertical bars (n 5 animals). Mean values with unlike letters were significantly different (p ≤ 0.05). AU = Arbitrary Units.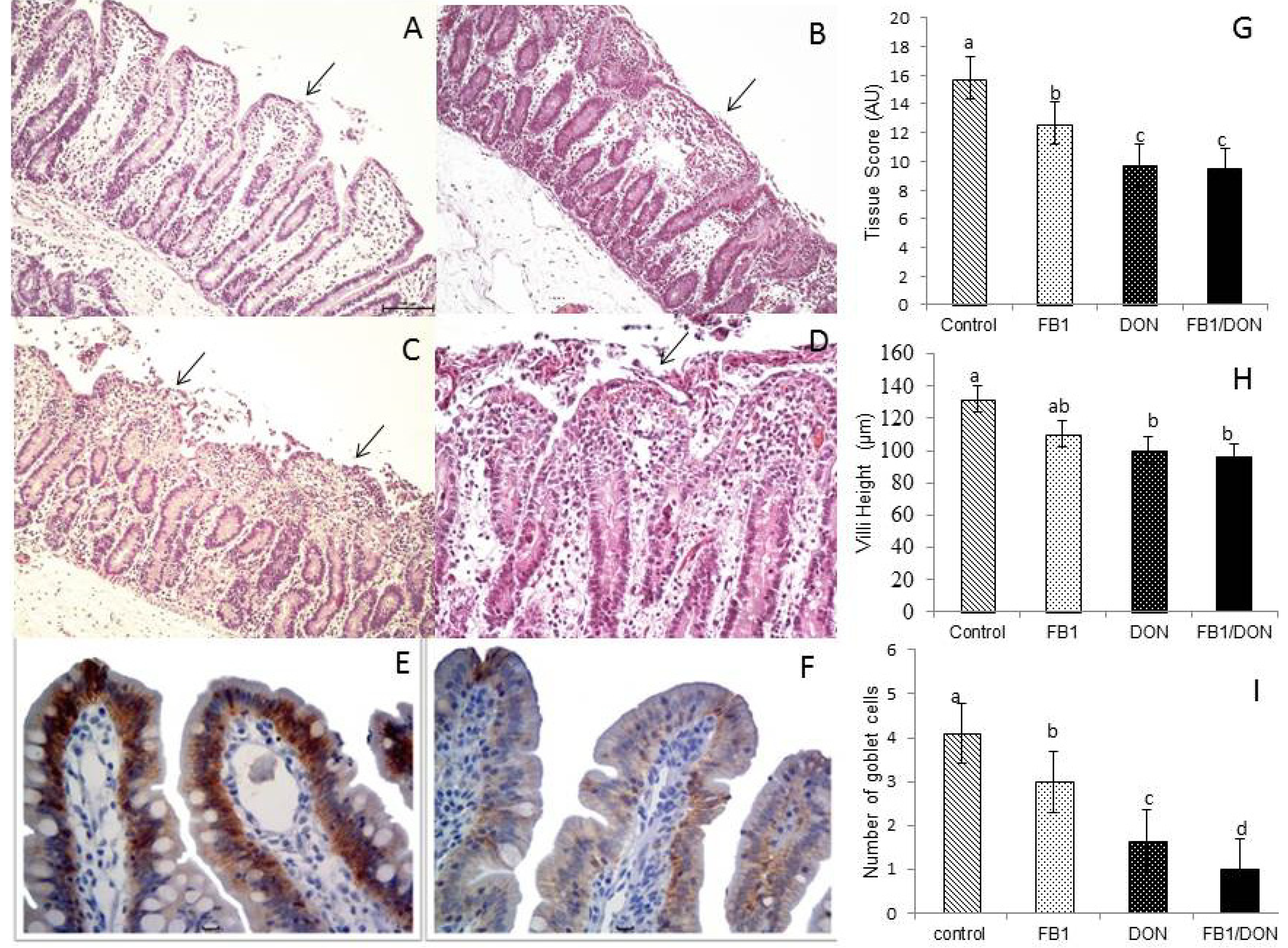
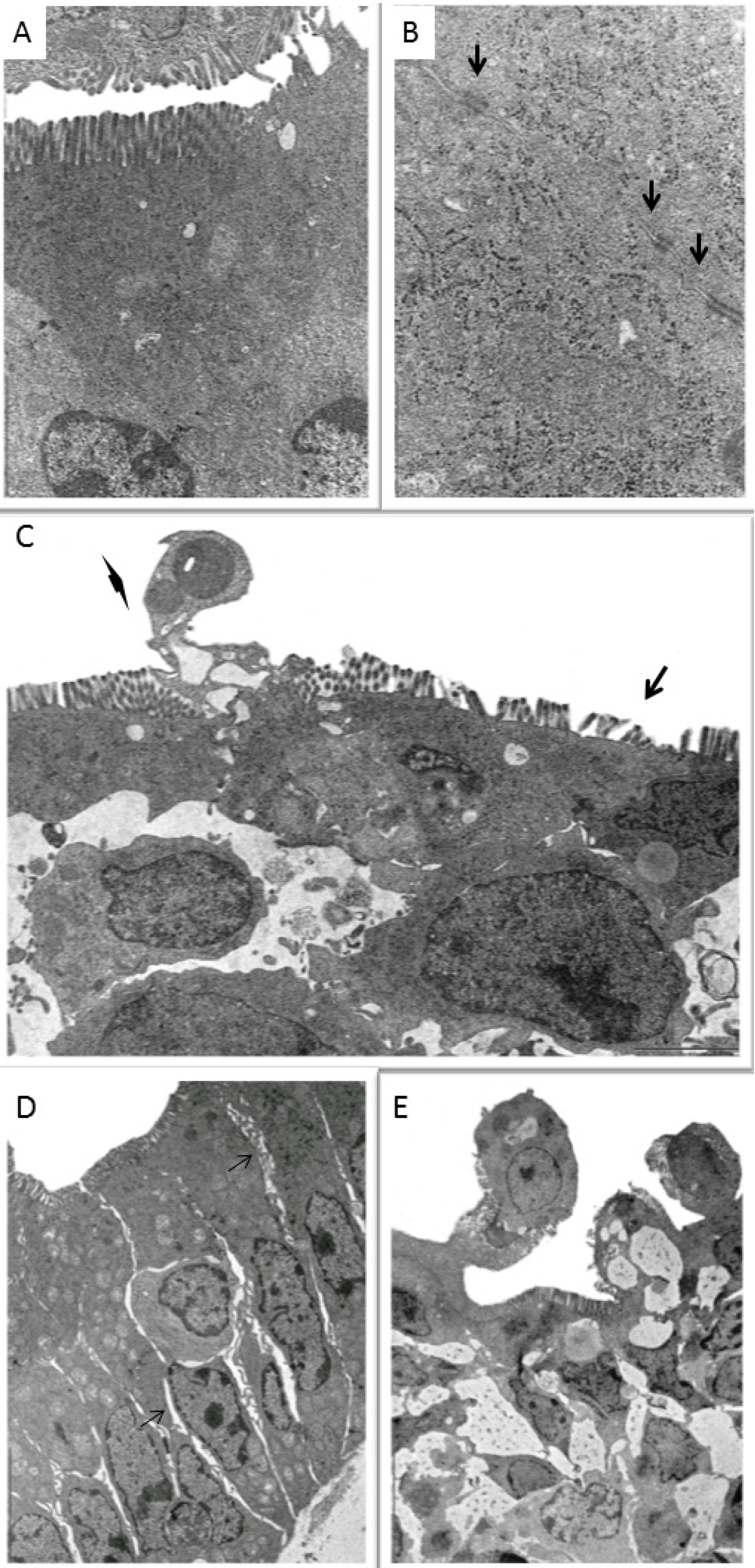
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Culture of Explants and Exposure to FB1 and DON
4.3. Histological and Morphometric Analysis
4.4. Immunohistochemical Analysis
4.5. Ultrastructural Analysis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cast, I. Mycotoxins—Risks in Plant, Animal and Human Systems; Task Force Report, No.139; Council for Agricultural Science and Technology: Ames, IA, USA, 2003; pp. 1–191.
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef]
- Dilkin, P.; Direito, G.; Simas, M.M.S.; Mallmann, C.A.; Corrêa, B. Toxicokinetics and toxicological effects of single oral dose of fumonisin B1 containing Fusarium verticillioides culture material in weaned piglets. Chemico-Biol. Interact. 2010, 185, 157–160. [Google Scholar] [CrossRef]
- Minami, L.; Meirelles, P.G.; Hirooka, E.Y.; Ono, E.Y.S. Fumonisinas: Efeitos toxicológicos, mecanismo de ação e biomarcadores para avaliação da exposição. Semina 2004, 25, 207–224. [Google Scholar]
- Bouhet, S.; Hourcade, E.; Loiseau, N.; Fikry, A.; Martinez, S.; Roselli, M.; Galtier, P.; Mengheri, E.; Oswald, I.P. The mycotoxin fumonisin B1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. 2004, 77, 165–171. [Google Scholar]
- Grenier, B.; Bracarense, A.P.F.R.L.; Lucioli, J.; Pacheco, G.D.; Cossalter, A.M.; Moll, W.D.; Schatzmayr, G.; Oswald, I.P. Individual and combined effects of subclinical doses of deoxynivalenol and fumonisin in piglets. Mol. Nutr. Food Res. 2011, 55, 761–771. [Google Scholar] [CrossRef]
- Bracarense, A.P.; Lucioli, J.; Grenier, B.; Pacheco, G.D.; Moll, W.D.; Schatzma, Y.R.G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef]
- Oswald, I.P.; Desautels, C.; Lafftte, J.; Fournout, S.; Peress, Y.; Odin, M.; Bars, L.E.; Bars, J.; Fairbrother, J.M. Mycotoxin fumonisin B1 increases intestinal colonization by pathogenic Escherichia coli in pigs. Appl. Environ. Microbiol. 2003, 69, 5870–5874. [Google Scholar]
- Maresca, M.; Yahi, N.; Younès-Sakr, L.; Boyron, M.; Caporiccio, B.; Fantini, J. Both direct and indirect effects account for the pro-inflammatory activity of enteropathogenic mycotoxins on the human intestinal epithelium: Stimulation of interleukin-8 secretion, potentiation of interleukin-1 β effect and increase in the transepithelial passage of commensal bacteria. Toxicol. Appl. Pharmacol. 2008, 22, 884–892. [Google Scholar]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Döll, S.; Dänicke, S. The fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) in animal feeding. Prev. Vet. Med. 2011, 102, 132–145. [Google Scholar] [CrossRef]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar]
- Maresca, M.; Fantini, J. Some food-associated mycotoxins as potential risk factors in humanspredisposed to chronic intestinal inflammatory diseases. Toxicon 2010, 56, 282–294. [Google Scholar] [CrossRef]
- Kolf-clauw, M.; Castellote, J.; Joly, B.; Bourges-Abella, N.; Raymond-Letron, I.; Pinton, P.; Oswald, I.P. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: Histopathological analysis. Toxicol. Vitro 2009, 23, 1580–1584. [Google Scholar] [CrossRef]
- Pinton, P.; Nougayrède, J.P.; Del Rio, J.C. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [Green Version]
- Pinton, P.; Braicu, C.; Nougayrede, J.; Lafitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar]
- Vandenbroucke, V.; Croubels, S.; Martel, A.; Verbrugghe, E.; Goossens, J.; Deun, K.V.; Boyen, F.; Thompson, A.; Shearer, N.; de Backer, P.; et al. The mycotoxin deoxynivalenol potentiates intestinal inflammation by Salmonella Typhimurium in porcine ileal loops. PloS One 2011, 6, e23871. [Google Scholar] [CrossRef] [Green Version]
- Witloc, D.R.; Waytt, R.D.; Ruff, M.D. Morphological changes in the avian intestine induced by citrinin and lack of effect of aflatoxin and T-2 toxin as seen with scanning electron microscopy. Toxicon 1977, 15, 41–44. [Google Scholar] [CrossRef]
- Obremski, K.; Gajecka, M.; Zielonka, L.; Jakimiuk, E.; Gajecki, M. Morphology and ultrastructure of small intestine mucosa in gilts with zearalenone mycotoxicosis. Pol. J. Vet. Sci. 2005, 8, 301–307. [Google Scholar]
- Lucioli, J.; Pinton, P.; Callu, P.; Laffitte, J.; Grosjean, F.; Kolf-Clauw, M.; Oswald, I.P.; Bracarense, A.P.F.R.L. The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: Interest of ex vivo models as an alternative to in vivo experiments. Toxicon 2013, 66, 31–36. [Google Scholar]
- Randall, K.; Turton, J.; Foster, J.R. Explant culture of gastrointestinal tissue: A review of methods and applications. Cell. Biol. Toxicol. 2011, 27, 267–284. [Google Scholar] [CrossRef]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef]
- Bouhet, S.; Oswald, I.P. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food. Res. 2007, 51, 925–931. [Google Scholar] [CrossRef]
- Lessard, M.; Boudry, G.; Sève, B.; Oswald, I.P.; Lallès, J.P. Intestinal physiology and peptidase activity in male pigs are modulated by consumption of corn culture extracts containing fumonisins. J. Nutr. 2009, 139, 1303–1307. [Google Scholar] [CrossRef]
- Zielonka, L.; Wiśniewska, M.; Obremski, K.; Gajęcki, M. Influence of low doses of deoxynivalenol on histopathology of selected organs of pigs. Pol. J. Vet. Sci. 2009, 12, 89–95. [Google Scholar]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.F.R.L.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junctions proteins and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef]
- Bae, H.K.; Pestka, J.J. Deoxynivalenol induces p38 interaction with the ribosome in monocytes and macrophages. Toxicol. Sci. 2008, 105, 59–66. [Google Scholar] [CrossRef]
- Voss, K.A.; Smith, G.M.; Hascheck, W.M. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Animal Feed Sci. Technol. 2007, 137, 299–325. [Google Scholar] [CrossRef]
- Dilkin, P.; Hassegawa, R.; Reis, T.A.; Mallmann, C.A.; Corrêa, B. Intoxicação experimental de suínos por fumonisinas. Cienc. Rural. 2004, 34, 175–191. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Brown, T.; Rottinghaus, G.; Williams, M. Fumonisin mycotoxicosis in broilers: Performances and pathology. Avian Diseases 1992, 36, 450–454. [Google Scholar]
- Goope, N.V.; Sharma, R.P.H. Fumonisin B1-induced apoptosis is associated with delayed inhibition of protein kinase C, nuclear factor-kappa B and tumor necrosis factor alpha in LLC-PK1 cells. Chem-Biol. Interact. 2003, 146, 131–145. [Google Scholar] [CrossRef]
- Gartner, L.P.; Hiatt, J.L. Sistema Digestivo. In Tratado de Histologia em cores, 3rd ed.; Elsevier: Rio de Janeiro, Brasil, 2007; pp. 387–416. [Google Scholar]
- De Walle, J.V.; Sergent, T.; Piront, N.; Toussaint, O.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010, 15, 291–298. [Google Scholar]
- Marin, D.E.; Gouze, M.E.; Taranu, I.; Oswald, I.P. Fumonisin B1 alters cell cycle progression and interleukin-2 synthesis in swine peripheral blood mononuclear cells. Mol. Nutr. Food Res. 2007, 51, 1406–1412. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Basso, K.; Gomes, F.; Bracarense, A.P.L. Deoxynivanelol and Fumonisin, Alone or in Combination, Induce Changes on Intestinal Junction Complexes and in E-Cadherin Expression. Toxins 2013, 5, 2341-2352. https://doi.org/10.3390/toxins5122341
Basso K, Gomes F, Bracarense APL. Deoxynivanelol and Fumonisin, Alone or in Combination, Induce Changes on Intestinal Junction Complexes and in E-Cadherin Expression. Toxins. 2013; 5(12):2341-2352. https://doi.org/10.3390/toxins5122341
Chicago/Turabian StyleBasso, Karina, Fernando Gomes, and Ana Paula Loureiro Bracarense. 2013. "Deoxynivanelol and Fumonisin, Alone or in Combination, Induce Changes on Intestinal Junction Complexes and in E-Cadherin Expression" Toxins 5, no. 12: 2341-2352. https://doi.org/10.3390/toxins5122341



