Immunometabolism in Obese Asthmatics: Are We There Yet?
Abstract
:1. Introduction
2. Immunometabolism in Obesity
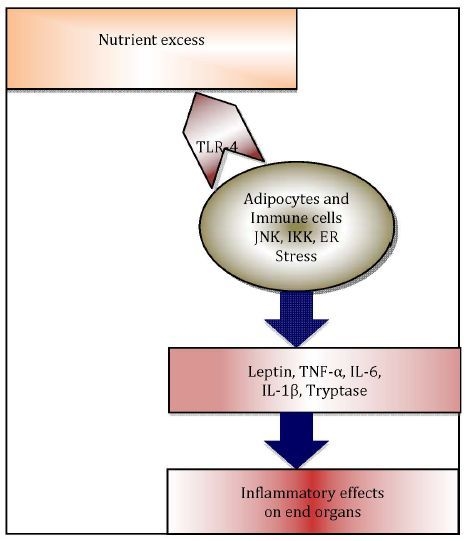
2.1. Metaflammation in Adipose Tissue
2.2. Adipokines

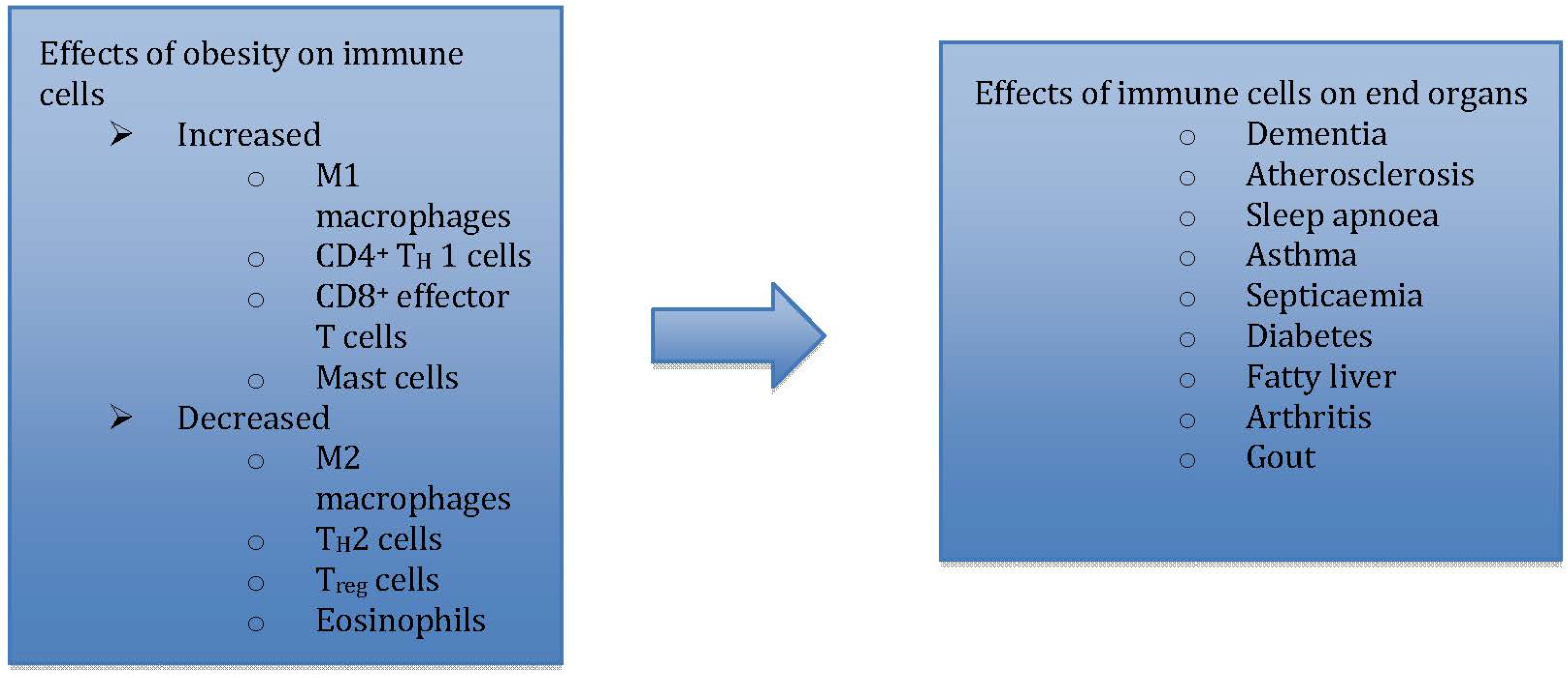
2.3. Macrophage Infiltration of Adipose Tissue
2.4. Macrophage Polarisation
2.5. Mast Cells
3. Immunometabolism in Obese Asthmatics
3.1. Adipokines
3.2. Macrophages
3.3. Mast Cells
4. Effect of Immunometabolism on Airway Inflammation in Obese Asthmatics
5. Therapeutic Possibilities
6. Conclusions
Acknowledgments
Conflicts of interest
References
- Au, N. The health care cost implications of overweight and obesity during childhood. Health Serv. Res. 2012, 47, 655–676. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, H.; Simental-Mendia, L.E.; Rodriguez-Ramirez, G.; Reyes-Romero, M.A. Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. Int. J. Endocrinol. 2013, 2013, 1–11. [Google Scholar]
- Block, J.P.; Subramanian, S.V.; Christakis, N.A.; O’Malley, A.J. Population trends and variation in body mass index from 1971 to 2008 in the framingham heart study offspring cohort. PLoS One 2013, 8, e63217. [Google Scholar]
- Ali, Z.; Ulrik, C.S. Obesity and asthma: A coincidence or a causal relationship? A systematic review. Respir. Med. 2013, 107, 1287–1300. [Google Scholar] [CrossRef]
- Mosen, D.M.; Schatz, M.; Magid, D.J.; Camargo, C.A., Jr. The relationship between obesity and asthma severity and control in adults. J. Allergy Clin. Immunol. 2008, 122, 507–511. [Google Scholar] [CrossRef]
- Ford, E.S. The epidemiology of obesity and asthma. J. Allergy Clin. Immunol. 2005, 115, 897–909. [Google Scholar] [CrossRef]
- Black, M.H.; Smith, N.; Porter, A.H.; Jacobsen, S.J.; Koebnick, C. Higher prevalence of obesity among children with asthma. Obesity (Silver Spring) 2012, 20, 1041–1047. [Google Scholar] [CrossRef]
- Brumpton, B.; Langhammer, A.; Romundstad, P.; Chen, Y.; Mai, X.M. General and abdominal obesity and incident asthma in adults: The HUNT study. Eur. Respir. J. 2013, 41, 323–329. [Google Scholar] [CrossRef]
- Saint-Pierre, P.; Bourdin, A.; Chanez, P.; Daures, J.P.; Godard, P. re overweight asthmatics more difficult to control? Allergy 2006, 61, 79–84. [Google Scholar]
- Stream, A.R.; Sutherland, E.R. Obesity and asthma disease phenotypes. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 76–81. [Google Scholar] [CrossRef]
- Lessard, A.; Turcotte, H.; Cormier, Y.; Boulet, L.P. Obesity and asthma: A specific phenotype? Chest 2008, 134, 317–323. [Google Scholar] [CrossRef]
- Baek, H.S.; Kim, Y.D.; Shin, J.H.; Kim, J.H.; Oh, J.W.; Lee, H.B. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann. Allergy Asthma Immunol. 2011, 107, 14–21. [Google Scholar] [CrossRef]
- Holguin, F.; Rojas, M.; Brown, L.A.; Fitzpatrick, A.M. Airway and plasma leptin and adiponectin in lean and obese asthmatics and controls. J. Asthma 2011, 48, 217–223. [Google Scholar] [CrossRef]
- Zerah, F.; Harf, A.; Perlemuter, L.; Lorino, H.; Lorino, A.M.; Atlan, G. Effects of obesity on respiratory resistance. Chest 1993, 103, 1470–1476. [Google Scholar] [CrossRef]
- Thomsen, S.F.; Ulrik, C.S.; Kyvik, K.O.; Sorensen, T.I.; Posthuma, D.; Skadhauge, L.R.; Steffensen, I.; Backer, V. Association between obesity and asthma in a twin cohort. Allergy 2007, 62, 1199–1204. [Google Scholar] [CrossRef]
- Melen, E.; Himes, B.E.; Brehm, J.M.; Boutaoui, N.; Klanderman, B.J.; Sylvia, J.S.; Lasky-Su, J. Analyses of shared genetic factors between asthma and obesity in children. J. Allergy Clin. Immunol. 2010, 126, 631–637. [Google Scholar] [CrossRef]
- Farah, C.S.; Salome, C.M. Asthma and obesity: A known association but unknown mechanism. Respirology 2012, 17, 412–421. [Google Scholar] [CrossRef]
- Mathis, D.; Shoelson, S.E. Immunometabolism: An emerging frontier. Nat. Rev. Immunol. 2011, 11. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Goforth, M.H.; Morel, C.R.; Subramanian, V.; Mukundan, L.; Red Eagle, A.; Vats, D.; Brombacher, F.; Ferrante, A.W.; et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007, 447, 1116–1120. [Google Scholar] [CrossRef]
- Divoux, A.; Moutel, S.; Poitou, C.; Lacasa, D.; Veyrie, N.; Aissat, A.; Arock, M.; Guerre-Millo, M.; Clement, K. Mast cells in human adipose tissue: Link with morbid obesity, inflammatory status, and diabetes. J. Clin. Endocrinol. Metab. 2012, 97, E1677–E1685. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Wang, T.N.; Wu, C.C.; Huang, M.S.; Wang, C.C.; Huang, C.C.; Wang, T.H.; Lien, T.C.; Ko, Y.C.; Lin, M.C. The polymorphisms of C-reactive protein gene modify the association between central obesity and lung function in taiwan asthmatics. Scand. J. Immunol. 2011, 74, 482–488. [Google Scholar] [CrossRef]
- Cheng, S.; Fox, C.S.; Larson, M.G.; Massaro, J.M.; McCabe, E.L.; Khan, A.M.; Levy, D.; Hoffmann, U.; O’Donnell, C.J.; Miller, K.K.; et al. Relation of visceral adiposity to circulating natriuretic peptides in ambulatory individuals. Am. J. Cardiol. 2011, 108, 979–984. [Google Scholar] [CrossRef]
- Li, C.; Ford, E.S.; McGuire, L.C.; Mokdad, A.H. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring) 2007, 15, 216–224. [Google Scholar] [CrossRef]
- Sideleva, O.; Suratt, B.T.; Black, K.E.; Tharp, W.G.; Pratley, R.E.; Forgione, P.; Dienz, O.; Irvin, C.G.; Dixon, A.E. Obesity and asthma: An inflammatory disease of adipose tissue not the airway. Am. J. Respir. Crit. Care Med. 2012, 186, 598–605. [Google Scholar] [CrossRef]
- Sood, A. Obesity, adipokines, and lung disease. J. Appl. Physiol. 2010, 108, 744–753. [Google Scholar] [CrossRef]
- Harwood, H.J., Jr. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 2011, 63, 57–75. [Google Scholar] [CrossRef]
- Fonseca-Alaniz, M.H.; Takada, J.; Alonso-Vale, M.I.; Lima, F.B. Adipose tissue as an endocrine organ: From theory to practice. J. Pediatr. (Rio J.) 2007, 83, 192–203. [Google Scholar] [CrossRef]
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or yperplasia: Dynamics of dipose tissue growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Yalcin, A.; Hotamisligil, G.S. Impact of ER protein homeostasis on metabolism. Diabetes 2013, 62, 691–693. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- De Nardo, D.; Latz, E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011, 32, 373–379. [Google Scholar] [CrossRef]
- Horng, T.; Hotamisligil, G.S. Linking the inflammasome to obesity-related disease. Nat. Med. 2011, 17, 164–165. [Google Scholar] [CrossRef]
- Stienstra, R.; Tack, C.J.; Kanneganti, T.D.; Joosten, L.A.; Netea, M.G. The inflammasome puts obesity in the danger zone. Cell Metab. 2012, 15, 10–18. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Calay, E.S.; Hotamisligil, G.S. Turning off the inflammatory, but not the metabolic, flames. Nat. Med. 2013, 19, 265–267. [Google Scholar] [CrossRef]
- Vachharajani, V.; Cunningham, C.; Yoza, B.; Carson, J., Jr.; Vachharajani, T.J.; McCall, C. Adiponectin-deficiency exaggerates sepsis-induced microvascular dysfunction in the mouse brain. Obesity (Silver Spring) 2012, 20, 498–504. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Role of adiponectin and PBEF/visfatin as regulators of inflammation: Involvement in obesity-associated diseases. Clin. Sci. (Lond.) 2008, 114, 275–288. [Google Scholar] [CrossRef]
- Zarkesh-Esfahani, H.; Pockley, A.G.; Wu, Z.; Hellewell, P.G.; Weetman, A.P.; Ross, R.J. Leptin indirectly activates human neutrophils via induction of TNF-alpha. J. Immunol. 2004, 172, 1809–1814. [Google Scholar]
- Considine, R.V.; Caro, J.F. Leptin and the regulation of body weight. Int. J. Biochem. Cell Biol. 1997, 29, 1255–1272. [Google Scholar] [CrossRef]
- Caro, J.F.; Sinha, M.K.; Kolaczynski, J.W.; Zhang, P.L.; Considine, R.V. Leptin: The tale of an obesity gene. Diabetes 1996, 45, 1455–1462. [Google Scholar]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995, 269, 540–543. [Google Scholar]
- Campfield, L.A.; Smith, F.J.; Guisez, Y.; Devos, R.; Burn, P. Recombinant mouse OB protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science 1995, 269, 546–549. [Google Scholar]
- Procaccini, C.; Jirillo, E.; Matarese, G. Leptin as an immunomodulator. Mol. Aspects Med. 2012, 33, 35–45. [Google Scholar] [CrossRef]
- Caldefie-Chezet, F.; Poulin, A.; Vasson, M.P. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic. Res. 2003, 37, 809–814. [Google Scholar] [CrossRef]
- Bouloumie, A.; Marumo, T.; Lafontan, M.; Busse, R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999, 13, 1231–1238. [Google Scholar]
- Allison, M.A.; Ix, J.H.; Morgan, C.; McClelland, R.L.; Rifkin, D.; Shimbo, D.; Criqui, M.H. Higher leptin is associated with hypertension: The multi-ethnic study of atherosclerosis. J. Hum. Hypertens. 2013. [Google Scholar] [CrossRef]
- Wang, H.; Luo, W.; Eitzman, D.T. Leptin in thrombosis and atherosclerosis. Curr. Pharm. Des. 2013, in press. [Google Scholar]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef]
- Kern, P.A.; Di Gregorio, G.B.; Lu, T.; Rassouli, N.; Ranganathan, G. Adiponectin expression from human adipose tissue: Relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes 2003, 52, 1779–1785. [Google Scholar] [CrossRef]
- Yang, W.S.; Lee, W.J.; Funahashi, T.; Tanaka, S.; Matsuzawa, Y.; Chao, C.L.; Chen, C.L.; Tai, T.Y.; Chuang, L.M. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J. Clin. Endocrinol. Metab. 2001, 86, 3815–3819. [Google Scholar] [CrossRef]
- Bruun, J.M.; Lihn, A.S.; Verdich, C.; Pedersen, S.B.; Toubro, S.; Astrup, A.; Richelsen, B. Regulation of adiponectin by adipose tissue-derived cytokines: In vivo and in vitro investigations in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E527–E533. [Google Scholar]
- Utsal, L.; Tillmann, V.; Zilmer, M.; Maestu, J.; Purge, P.; Jurimae, J.; Saar, M.; Latt, E.; Maasalu, K.; Jurimae, T. Elevated serum IL-6, IL-8, MCP-1, CRP, and IFN-gamma levels in 10- to 11-year-old boys with increased BMI. Horm. Res. Paediatr. 2012, 78, 31–39. [Google Scholar] [CrossRef]
- Ajuwon, K.M.; Spurlock, M.E. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J. Nutr. 2005, 135, 1841–1846. [Google Scholar]
- Cook, D.G.; Mendall, M.A.; Whincup, P.H.; Carey, I.M.; Ballam, L.; Morris, J.E.; Miller, G.J.; Strachan, D.P. C-reactive protein concentration in children: Relationship to adiposity and other cardiovascular risk factors. Atherosclerosis 2000, 149, 139–150. [Google Scholar] [CrossRef]
- Warnberg, J.; Moreno, L.A.; Mesana, M.I.; Marcos, A. Inflammatory mediators in overweight and obese Spanish adolescents. The AVENA Study. Int. J. Obes. Relat. Metab. Disord. 2004, 28 (Suppl. 3), 59–63. [Google Scholar] [CrossRef]
- Rawson, E.S.; Freedson, P.S.; Osganian, S.K.; Matthews, C.E.; Reed, G.; Ockene, I.S. Body mass index, but not physical activity, is associated with C-reactive protein. Med. Sci. Sports Exerc. 2003, 35, 1160–1166. [Google Scholar] [CrossRef]
- Rommel, J.; Simpson, R.; Mounsey, J.P.; Chung, E.; Schwartz, J.; Pursell, I.; Gehi, A. Effect of body mass index, physical activity, depression, and educational attainment on high-sensitivity C-reactive protein in patients with atrial fibrillation. Am. J. Cardiol. 2013, 111, 208–212. [Google Scholar]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Suganami, T.; Ogawa, Y. Adipose tissue macrophages: Their role in adipose tissue remodeling. J. Leukoc. Biol. 2010, 88, 33–39. [Google Scholar] [CrossRef]
- Cancello, R.; Tordjman, J.; Poitou, C.; Guilhem, G.; Bouillot, J.L.; Hugol, D.; Coussieu, C.; Basdevant, A.; Bar Hen, A.; Bedossa, P.; et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 2006, 55, 1554–1561. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Seo, K.; Yamashita, H.; Hosoya, Y.; Ohsugi, M.; Tobe, K.; Kadowaki, T.; Nagai, R.; et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J. Clin. Investig. 2008, 118, 710–721. [Google Scholar]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar]
- Riordan, N.H.; Ichim, T.E.; Min, W.P.; Wang, H.; Solano, F.; Lara, F.; Alfaro, M.; Rodriguez, J.P.; Harman, R.J.; Patel, A.N.; et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J. Transl. Med. 2009, 7. [Google Scholar] [CrossRef]
- Schipper, H.S.; Prakken, B.; Kalkhoven, E.; Boes, M. Adipose tissue-resident immune cells: Key players in immunometabolism. Trends Endocrinol. Metab. 2012, 23, 407–415. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Strissel, K.J.; Stancheva, Z.; Miyoshi, H.; Perfield, J.W., II; DeFuria, J.; Jick, Z.; Greenberg, A.S.; Obin, M.S. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007, 56, 2910–2918. [Google Scholar] [CrossRef]
- Alkhouri, N.; Gornicka, A.; Berk, M.P.; Thapaliya, S.; Dixon, L.J.; Kashyap, S.; Schauer, P.R.; Feldstein, A.E. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J. Biol. Chem. 2010, 285, 3428–3438. [Google Scholar] [CrossRef]
- Tordjman, J.; Poitou, C.; Hugol, D.; Bouillot, J.L.; Basdevant, A.; Bedossa, P.; Guerre-Millo, M.; Clement, K. Association between omental adipose tissue macrophages and liver histopathology in morbid obesity: Influence of glycemic status. J. Hepatol. 2009, 51, 354–362. [Google Scholar] [CrossRef]
- Abreu Velez, A.M.; Dejoseph, L.M.; Howard, M.S. HAM56 and CD68 antigen presenting cells surrounding a sarcoidal granulomatous tattoo. N. Am. J. Med. Sci. 2011, 3, 475–477. [Google Scholar]
- Sethi, J.K.; Vidal-Puig, A.J. Thematic review series: Adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 2007, 48, 1253–1262. [Google Scholar] [CrossRef]
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-Associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar]
- Chinetti-Gbaguidi, G.; Staels, B. Macrophage polarization in metabolic disorders: Functions and regulation. Curr. Opin. Lipidol. 2011, 22, 365–372. [Google Scholar] [CrossRef]
- Cairo, G.; Recalcati, S.; Mantovani, A.; Locati, M. Iron trafficking and metabolism in macrophages: Contribution to the polarized phenotype. Trends Immunol. 2011, 32, 241–247. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar]
- Rodriguez-Prados, J.C.; Traves, P.G.; Cuenca, J.; Rico, D.; Aragones, J.; Martin-Sanz, P.; Cascante, M.; Bosca, L. Substrate fate in activated macrophages: A comparison between innate, classic, and alternative activation. J. Immunol. 2010, 185, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Van Gool, F.; Galli, M.; Gueydan, C.; Kruys, V.; Prevot, P.P.; Bedalov, A.; Mostoslavsky, R.; Alt, F.W.; de Smedt, T.; Leo, O. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat. Med. 2009, 15, 206–210. [Google Scholar] [CrossRef]
- Van den Heuvel, M.M.; Tensen, C.P.; van As, J.H.; van den Berg, T.K.; Fluitsma, D.M.; Dijkstra, C.D.; Dopp, E.A.; Droste, A.; van Gaalen, F.A.; Sorg, C.; et al. Regulation of CD 163 on human macrophages: Cross-Linking of CD163 induces signaling and activation. J. Leukoc. Biol. 1999, 66, 858–866. [Google Scholar]
- Moller, H.J. Soluble CD163. Scand. J. Clin. Lab. Investig. 2012, 72, 1–13. [Google Scholar] [CrossRef]
- Buechler, C.; Ritter, M.; Orso, E.; Langmann, T.; Klucken, J.; Schmitz, G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 2000, 67, 97–103. [Google Scholar]
- Burdo, T.H.; Lo, J.; Abbara, S.; Wei, J.; DeLelys, M.E.; Preffer, F.; Rosenberg, E.S.; Williams, K.C.; Grinspoon, S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J. Infect. Dis. 2011, 204, 1227–1236. [Google Scholar] [CrossRef]
- Knudsen, T.B.; Larsen, K.; Kristiansen, T.B.; Moller, H.J.; Tvede, M.; Eugen-Olsen, J.; Kronborg, G. Diagnostic value of soluble CD163 serum levels in patients suspected of meningitis: Comparison with CRP and procalcitonin. Scand. J. Infect. Dis. 2007, 39, 542–553. [Google Scholar] [CrossRef]
- Fjeldborg, K.; Christiansen, T.; Bennetzen, M.; Moller, H.J.; Pedersen, S.B.; Richelsen, B. The macrophage specific serum marker, soluble CD163, is increased in obesity and reduced after dietary induced weight loss. Obesity (Silver Spring) 2013. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Bindahman, L.S.; Alokail, M.S.; Alkharfy, K.M.; Draz, H.M.; Yakout, S.; McTernan, P.G.; Sabico, S.; Chrousos, G.P. Soluble CD163 is associated with body mass index and blood pressure in hypertensive obese Saudi patients. Eur. J. Clin. Investig. 2012, 42, 1221–1226. [Google Scholar] [CrossRef]
- Moestrup, S.K.; Moller, H.J. CD163: A regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann. Med. 2004, 36, 347–354. [Google Scholar] [CrossRef]
- Van Gorp, H.; Delputte, P.L.; Nauwynck, H.J. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol. Immunol. 2010, 47, 1650–1660. [Google Scholar] [CrossRef]
- Fabriek, B.O.; Dijkstra, C.D.; van den Berg, T.K. The macrophage scavenger receptor CD163. Immunobiology 2005, 210, 153–160. [Google Scholar] [CrossRef]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Backe, E.; Schwarting, R.; Gerdes, J.; Ernst, M.; Stein, H. Ber-MAC3: New monoclonal antibody that defines human monocyte/macrophage differentiation antigen. J. Clin. Pathol. 1991, 44, 936–945. [Google Scholar]
- Parkner, T.; Sorensen, L.P.; Nielsen, A.R.; Fischer, C.P.; Bibby, B.M.; Nielsen, S.; Pedersen, B.K.; Moller, H.J. Soluble CD163: A biomarker linking macrophages and insulin resistance. Diabetologia 2012, 55, 1856–1862. [Google Scholar] [CrossRef]
- Yin, M.J.; Yamamoto, Y.; Gaynor, R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 1998, 396, 77–80. [Google Scholar] [CrossRef]
- Apovian, C.M.; Bigornia, S.; Mott, M.; Meyers, M.R.; Ulloor, J.; Gagua, M.; McDonnell, M.; Hess, D.; Joseph, L.; Gokce, N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1654–1659. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar]
- Axelsson, J.; Moller, H.J.; Witasp, A.; Qureshi, A.R.; Carrero, J.J.; Heimburger, O.; Barany, P.; Alvestrand, A.; Lindholm, B.; Moestrup, S.K.; et al. Changes in fat mass correlate with changes in soluble sCD163, a marker of mature macrophages, in patients with CKD. Am. J. Kidney Dis. 2006, 48, 916–925. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar]
- Liu, J.; Divoux, A.; Sun, J.; Zhang, J.; Clement, K.; Glickman, J.N.; Sukhova, G.K.; Wolters, P.J.; Du, J.; Gorgun, C.Z.; et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 2009, 15, 940–945. [Google Scholar] [CrossRef]
- Liu, J.; Divoux, A.; Sun, J.; Zhang, J.; Clement, K.; Glickman, J.N.; Sukhova, G.K.; Wolters, P.J.; Du, J.; Gorgun, C.Z.; et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 2009, 15, 940–945. [Google Scholar] [CrossRef]
- Payne, V.; Kam, P.C. Mast cell tryptase: A review of its physiology and clinical significance. Anaesthesia 2004, 59, 695–703. [Google Scholar] [CrossRef]
- Komarow, H.D.; Hu, Z.; Brittain, E.; Uzzaman, A.; Gaskins, D.; Metcalfe, D.D. Serum tryptase levels in atopic and nonatopic children. J. Allergy Clin. Immunol. 2009, 124, 845–848. [Google Scholar] [CrossRef]
- Gonzalez-Quintela, A.; Vizcaino, L.; Gude, F.; Rey, J.; Meijide, L.; Fernandez-Merino, C.; Linneberg, A.; Vidal, C. Factors influencing serum total tryptase concentrations in a general adult population. Clin. Chem. Lab. Med. 2010, 48, 701–706. [Google Scholar]
- Fenger, R.V.; Linneberg, A.; Vidal, C.; Vizcaino, L.; Husemoen, L.L.; Aadahl, M.; Gonzalez-Quintela, A. Determinants of serum tryptase in a general population: The relationship of serum tryptase to obesity and asthma. Int. Arch. Allergy Immunol. 2012, 157, 151–158. [Google Scholar] [CrossRef]
- Seidell, J.C.; de Groot, L.C.; van Sonsbeek, J.L.; Deurenberg, P.; Hautvast, J.G. Associations of moderate and severe overweight with self-reported illness and medical care in Dutch adults. Am. J. Public Health 1986, 76, 264–269. [Google Scholar] [CrossRef]
- Negri, E.; Pagano, R.; Decarli, A.; La Vecchia, C. Body weight and the prevalence of chronic diseases. J. Epidemiol. Community Health 1988, 42, 24–29. [Google Scholar] [CrossRef]
- Shaheen, S.O.; Sterne, J.A.; Montgomery, S.M.; Azima, H. Birth weight, body mass index and asthma in young adults. Thorax 1999, 54, 396–402. [Google Scholar] [CrossRef]
- Beuther, D.A.; Sutherland, E.R. Overweight, obesity, and incident asthma: A meta-analysis of prospective epidemiologic studies. Am. J. Respir. Crit. Care Med. 2007, 175, 661–666. [Google Scholar] [CrossRef]
- Chen, Y.; Dales, R.; Tang, M.; Krewski, D. Obesity may increase the incidence of asthma in women but not in men: Longitudinal observations from the Canadian National Population Health Surveys. Am. J. Epidemiol. 2002, 155, 191–197. [Google Scholar] [CrossRef]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef]
- Camargo, C.A., Jr.; Weiss, S.T.; Zhang, S.; Willett, W.C.; Speizer, F.E. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch. Intern. Med. 1999, 159, 2582–2588. [Google Scholar] [CrossRef]
- Gold, D.R.; Damokosh, A.I.; Dockery, D.W.; Berkey, C.S. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatr. Pulmonol. 2003, 36, 514–521. [Google Scholar] [CrossRef]
- Bergen, H.T.; Cherlet, T.C.; Manuel, P.; Scott, J.E. Identification of leptin receptors in lung and isolated fetal type II cells. Am. J. Respir. Cell Mol. Biol. 2002, 27, 71–77. [Google Scholar] [CrossRef]
- Bruno, A.; Pace, E.; Chanez, P.; Gras, D.; Vachier, I.; Chiappara, G.; La Guardia, M.; Gerbino, S.; Profita, M.; Gjomarkaj, M. Leptin and leptin receptor expression in asthma. J. Allergy Clin. Immunol. 2009, 124, 230–237. [Google Scholar] [CrossRef]
- Hug, C.; Wang, J.; Ahmad, N.S.; Bogan, J.S.; Tsao, T.S.; Lodish, H.F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. USA 2004, 101, 10308–10313. [Google Scholar] [CrossRef]
- Mancuso, P.; Huffnagle, G.B.; Olszewski, M.A.; Phipps, J.; Peters-Golden, M. Leptin corrects host defense defects after acute starvation in murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 2006, 173, 212–218. [Google Scholar] [CrossRef]
- Sideleva, O.; Suratt, B.T.; Black, K.E.; Tharp, W.G.; Pratley, R.E.; Forgione, P.; Dienz, O.; Irvin, C.G.; Dixon, A.E. Obesity and Asthma: An Inflammatory Disease of Adipose Tissue Not the Airway. Am. J. Respir. Crit. Care Med. 2012, 186, 598–605. [Google Scholar] [CrossRef]
- Kim, K.W.; Shin, Y.H.; Lee, K.E.; Kim, E.S.; Sohn, M.H.; Kim, K.E. Relationship between adipokines and manifestations of childhood asthma. Pediatr. Allergy Immunol. 2008, 19, 535–540. [Google Scholar] [CrossRef]
- Sood, A.; Ford, E.S.; Camargo, C.A., Jr. Association between leptin and asthma in adults. Thorax 2006, 61, 300–305. [Google Scholar] [CrossRef]
- Sood, A.; Cui, X.; Qualls, C.; Beckett, W.S.; Gross, M.D.; Steffes, M.W.; Smith, L.J.; Jacobs, D.R., Jr. Association between asthma and serum adiponectin concentration in women. Thorax 2008, 63, 877–882. [Google Scholar] [CrossRef]
- Schols, A.M.; Creutzberg, E.C.; Buurman, W.A.; Campfield, L.A.; Saris, W.H.; Wouters, E.F. Plasma leptin is related to proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, 1220–1226. [Google Scholar] [CrossRef]
- Guler, N.; Kirerleri, E.; Ones, U.; Tamay, Z.; Salmayenli, N.; Darendeliler, F. Leptin: Does it have any role in childhood asthma? J. Allergy Clin. Immunol. 2004, 114, 254–259. [Google Scholar] [CrossRef]
- Johnston, R.A.; Schwartzman, I.N.; Shore, S.A. Macrophage inflammatory protein-2 levels are associated with changes in serum leptin concentrations following ozone-induced airway inflammation. Chest 2003, 123, 369S–370S. [Google Scholar] [CrossRef]
- Wood, L.G.; Gibson, P.G. Adiponectin: The link between obesity and asthma in women? Am. J. Respir. Crit. Care Med. 2012, 186, 1–2. [Google Scholar] [CrossRef]
- Tsaroucha, A.; Daniil, Z.; Malli, F.; Georgoulias, P.; Minas, M.; Kostikas, K.; Bargiota, A.; Zintzaras, E.; Gourgoulianis, K.I. Leptin, adiponectin, and ghrelin levels in female patients with asthma during stable and exacerbation periods. J. Asthma 2013, 50, 188–197. [Google Scholar] [CrossRef]
- Shore, S.A.; Terry, R.D.; Flynt, L.; Xu, A.; Hug, C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J. Allergy Clin. Immunol. 2006, 118, 389–395. [Google Scholar] [CrossRef]
- Summer, R.; Little, F.F.; Ouchi, N.; Takemura, Y.; Aprahamian, T.; Dwyer, D.; Fitzsimmons, K.; Suki, B.; Parameswaran, H.; Fine, A.; et al. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L1035–L1042. [Google Scholar] [CrossRef]
- Sood, A.; Ford, E.S.; Camargo, C.A., Jr. Association between leptin and asthma in adults. Thorax 2006, 61, 300–305. [Google Scholar] [CrossRef]
- Sood, A.; Cui, X.; Qualls, C.; Beckett, W.S.; Gross, M.D.; Steffes, M.W.; Smith, L.J.; Jacobs, D.R., Jr. Association between asthma and serum adiponectin concentration in women. Thorax 2008, 63, 877–882. [Google Scholar] [CrossRef]
- Sood, A.; Dominic, E.; Qualls, C.; Steffes, M.W.; Thyagarajan, B.; Smith, L.J.; Lewis, C.E.; Jacobs, D.R., Jr. Serum adiponectin is associated with adverse outcomes of asthma in men but not in women. Front. Pharmacol. 2011, 2. [Google Scholar] [CrossRef]
- Sato, H.; Sugai, H.; Kurosaki, H.; Ishikawa, M.; Funaki, A.; Kimura, Y.; Ueno, K. The effect of sex hormones on peroxisome proliferator-activated receptor gamma expression and activity in mature adipocytes. Biol. Pharm. Bull. 2013, 36, 564–573. [Google Scholar] [CrossRef]
- Wildman, R.P.; Wang, D.; Fernandez, I.; Mancuso, P.; Santoro, N.; Scherer, P.E.; Sowers, M.R. Associations of testosterone and sex hormone binding globulin with adipose tissue hormones in midlife women. Obesity (Silver Spring) 2013, 21, 629–636. [Google Scholar] [CrossRef]
- Zanni, M.V.; Burdo, T.H.; Makimura, H.; Williams, K.C.; Grinspoon, S.K. Relationship between monocyte/macrophage activation marker soluble CD163 and insulin resistance in obese and normal-weight subjects. Clin. Endocrinol. (Oxf.) 2012, 77, 385–390. [Google Scholar] [CrossRef]
- Sismanopoulos, N.; Delivanis, D.A.; Mavrommati, D.; Hatziagelaki, E.; Conti, P.; Theoharides, T.C. Do mast cells link obesity and asthma? Allergy 2012, 68, 8–15. [Google Scholar]
- Brightling, C.E.; Bradding, P.; Symon, F.A.; Holgate, S.T.; Wardlaw, A.J.; Pavord, I.D. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002, 346, 1699–1705. [Google Scholar] [CrossRef]
- Hogan, A.D.; Schwartz, L.B. Markers of mast cell degranulation. Methods 1997, 13, 43–52. [Google Scholar] [CrossRef]
- Sutcliffe, A.; Kaur, D.; Page, S.; Woodman, L.; Armour, C.L.; Baraket, M.; Bradding, P.; Hughes, J.M.; Brightling, C.E. Mast cell migration to Th2 stimulated airway smooth muscle from asthmatics. Thorax 2006, 61, 657–662. [Google Scholar] [CrossRef]
- Balzar, S.; Fajt, M.L.; Comhair, S.A.; Erzurum, S.C.; Bleecker, E.; Busse, W.W.; Castro, M.; Gaston, B.; Israel, E.; Schwartz, L.B.; et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2011, 183, 299–309. [Google Scholar] [CrossRef]
- Bradding, P.; Walls, A.F.; Holgate, S.T. The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol. 2006, 117, 1277–1284. [Google Scholar] [CrossRef]
- Brown, J.K.; Jones, C.A.; Tyler, C.L.; Ruoss, S.J.; Hartmann, T.; Caughey, G.H. Tryptase-induced mitogenesis in airway smooth muscle cells. Potency, mechanisms, and interactions with other mast cell mediators. Chest 1995, 107, 95S–96S. [Google Scholar] [CrossRef]
- Panettieri, R.A.; Tan, E.M.; Ciocca, V.; Luttmann, M.A.; Leonard, T.B.; Hay, D.W. Effects of LTD4 on human airway smooth muscle cell proliferation, matrix expression, and contraction in vitro: Differential sensitivity to cysteinyl leukotriene receptor antagonistS. Am. J. Respir. Cell Mol. Biol. 1998, 19, 453–461. [Google Scholar] [CrossRef]
- Gibson, P.G.; Saltos, N.; Borgas, T. Airway mast cells and eosinophils correlate with clinical severity and airway hyperresponsiveness in corticosteroid-treated asthma. J. Allergy Clin. Immunol. 2000, 105, 752–759. [Google Scholar] [CrossRef]
- Koshino, T.; Teshima, S.; Fukushima, N.; Takaishi, T.; Hirai, K.; Miyamoto, Y.; Arai, Y.; Sano, Y.; Ito, K.; Morita, Y. Identification of basophils by immunohistochemistry in the airways of post-mortem cases of fatal asthma. Clin. Exp. Allergy 1993, 23, 919–925. [Google Scholar] [CrossRef]
- Carroll, N.G.; Mutavdzic, S.; James, A.L. Increased mast cells and neutrophils in submucosal mucous glands and mucus plugging in patients with asthma. Thorax 2002, 57, 677–682. [Google Scholar] [CrossRef]
- Wood, L.G.; Gibson, P.G. Dietary factors lead to innate immune activation in asthma. Pharmacol. Ther. 2009, 123, 37–53. [Google Scholar] [CrossRef]
- Kharitonov, S.A.; Yates, D.; Springall, D.R.; Buttery, L.; Polak, J.; Robbins, R.A.; Barnes, P.J. Exhaled nitric oxide is increased in asthma. Chest 1995, 107, 156S–157S. [Google Scholar] [CrossRef]
- Kazaks, A.; Uriu-Adams, J.Y.; Stern, J.S.; Albertson, T.E. No significant relationship between exhaled nitric oxide and body mass index in people with asthma. J. Allergy Clin. Immunol. 2005, 116, 929–930. [Google Scholar] [CrossRef]
- Leung, T.F.; Li, C.Y.; Lam, C.W.; Au, C.S.; Yung, E.; Chan, I.H.; Wong, G.W.; Fok, T.F. The relation between obesity and asthmatic airway inflammation. Pediatr. Allergy Immunol. 2004, 15, 344–350. [Google Scholar] [CrossRef]
- Van Veen, I.H.; Ten Brinke, A.; Sterk, P.J.; Rabe, K.F.; Bel, E.H. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy 2008, 63, 570–574. [Google Scholar] [CrossRef]
- Jensen, M.E.; Collins, C.E.; Gibson, P.G.; Wood, L.G. The obesity phenotype in children with asthma. Paediatr. Respir. Rev. 2011, 12, 152–159. [Google Scholar] [CrossRef]
- Von Mutius, E.; Schwartz, J.; Neas, L.M.; Dockery, D.; Weiss, S.T. Relation of body mass index to asthma and atopy in children: The National Health and Nutrition Examination Study III. Thorax 2001, 56, 835–838. [Google Scholar] [CrossRef]
- Hashimoto, C.; Hudson, K.L.; Anderson, K.V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 1988, 52, 269–279. [Google Scholar] [CrossRef]
- Ghanim, H.; Sia, C.L.; Upadhyay, M.; Korzeniewski, K.; Viswanathan, P.; Abuaysheh, S.; Mohanty, P.; Dandona, P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am. J. Clin. Nutr. 2010, 91, 940–949. [Google Scholar] [CrossRef]
- Patel, C.; Ghanim, H.; Ravishankar, S.; Sia, C.L.; Viswanathan, P.; Mohanty, P.; Dandona, P. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J. Clin. Endocrinol. Metab. 2007, 92, 4476–4479. [Google Scholar] [CrossRef]
- Blackburn, P.; Despres, J.P.; Lamarche, B.; Tremblay, A.; Bergeron, J.; Lemieux, I.; Couillard, C. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity (Silver Spring) 2006, 14, 1747–1754. [Google Scholar] [CrossRef]
- Wiesner, P.; Choi, S.H.; Almazan, F.; Benner, C.; Huang, W.; Diehl, C.J.; Gonen, A.; Butler, S.; Witztum, J.L.; Glass, C.K.; et al. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: Possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ. Res. 2010, 107, 56–65. [Google Scholar] [CrossRef]
- Rosenkranz, S.K.; Townsend, D.K.; Steffens, S.E.; Harms, C.A. Effects of a high-fat meal on pulmonary function in healthy subjects. Eur. J. Appl. Physiol. 2010, 109, 499–506. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Gibson, P.G. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J. Allergy Clin. Immunol. 2011, 127, 1133–1140. [Google Scholar] [CrossRef]
- Zhao, L.; Kwon, M.J.; Huang, S.; Lee, J.Y.; Fukase, K.; Inohara, N.; Hwang, D.H. Differential modulation of Nods signaling pathways by fatty acids in human colonic epithelial HCT116 cells. J. Biol. Chem. 2007, 282, 11618–11628. [Google Scholar]
- Wood, L.G.; Garg, M.L.; Smart, J.M.; Scott, H.A.; Barker, D.; Gibson, P.G. Manipulating antioxidant intake in asthma: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 534–543. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Powell, H.; Gibson, P.G. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: Proof of concept. Free Radic. Res. 2008, 42, 94–102. [Google Scholar] [CrossRef]
- Todd, D.C.; Armstrong, S.; D’Silva, L.; Allen, C.J.; Hargreave, F.E.; Parameswaran, K. Effect of obesity on airway inflammation: A cross-sectional analysis of body mass index and sputum cell counts. Clin. Exp. Allergy 2007, 37, 1049–1054. [Google Scholar] [CrossRef]
- Wood, L.G.; Baines, K.J.; Fu, J.; Scott, H.A.; Gibson, P.G. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest 2012, 142, 86–93. [Google Scholar] [CrossRef]
- Telenga, E.D.; Tideman, S.W.; Kerstjens, H.A.; Hacken, N.H.; Timens, W.; Postma, D.S.; van den Berge, M. Obesity in asthma: More neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy 2012, 67, 1060–1068. [Google Scholar] [CrossRef]
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Wood, L.G. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur. Respir. J. 2011, 38, 594–602. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Swern, A.; Bird, S.S.; Hustad, C.M.; Grant, E.; Edelman, J.M. Influence of body mass index on the response to asthma controller agents. Eur. Respir. J. 2006, 27, 495–503. [Google Scholar] [CrossRef]
- Boulet, L.P.; Franssen, E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir. Med. 2007, 101, 2240–2247. [Google Scholar] [CrossRef]
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Wood, L.G. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur. Respir. J. 2011, 38, 594–602. [Google Scholar] [CrossRef]
- McLachlan, C.R.; Poulton, R.; Car, G.; Cowan, J.; Filsell, S.; Greene, J.M.; Taylor, D.R.; Welch, D.; Williamson, A.; Sears, M.R.; et al. Adiposity, asthma, and airway inflammation. J. Allergy Clin. Immunol. 2007, 119, 634–639. [Google Scholar] [CrossRef]
- Woods, S.C.; Gotoh, K.; Clegg, D.J. Gender differences in the control of energy homeostasis. Exp. Biol. Med. 2003, 228, 1175–1180. [Google Scholar]
- Van Harmelen, V.; Reynisdottir, S.; Eriksson, P.; Thörne, A.; Hoffstedt, J.; Lönnqvist, F.; Arner, P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998, 47, 913–917. [Google Scholar] [CrossRef]
- Troisi, R.J.; Speizer, F.E.; Willett, W.C.; Trichopoulos, D.; Rosner, B. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am. J. Respir. Crit. Care Med. 1995, 152, 1183–1188. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Holguin, F.; Teague, W.G.; Brown, L.A. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J. Allergy Clin. Immunol. 2008, 121, 1372–1378.e3. [Google Scholar] [CrossRef]
- Marguet, C.; Jouen-Boedes, F.; Dean, T.P.; Warner, J.O. Bronchoalveolar cell profiles in children with asthma, infantile wheeze, chronic cough, or cystic fibrosis. Am. J. Respir. Crit. Care Med. 1999, 159, 1533–1540. [Google Scholar] [CrossRef]
- Lugogo, N.L.; Hollingsworth, J.W.; Howell, D.L.; Que, L.G.; Francisco, D.; Church, T.D.; Potts-Kant, E.N.; Ingram, J.L.; Wang, Y.; Jung, S.H.; Kraft, M. Alveolar macrophages from overweight/obese subjects with asthma demonstrate a proinflammatory phenotype. Am. J. Respir. Crit. Care Med. 2012, 186, 404–411. [Google Scholar] [CrossRef]
- Lugogo, N.L.; Hollingsworth, J.W.; Howell, D.L.; Que, L.G.; Francisco, D.; Church, T.D.; Potts-Kant, E.N.; Ingram, J.L.; Wang, Y.; Jung, S.H.; et al. Alveolar macrophages from overweight/obese subjects with asthma demonstrate a proinflammatory phenotype. Am. J. Respir. Crit. Care Med. 2012, 186, 404–411. [Google Scholar] [CrossRef]
- Fernandez-Boyanapalli, R.; Goleva, E.; Kolakowski, C.; Min, E.; Day, B.; Leung, D.Y.; Riches, D.W.; Bratton, D.L.; Sutherland, E.R. Obesity impairs apoptotic cell clearance in asthma. J. Allergy Clin. Immunol. 2013, 131, 1041–1047.e3. [Google Scholar] [CrossRef]
- Zhang, Y.; Berger, A.; Milne, C.D.; Paige, C.J. Tachykinins in the immune system. Curr. Drug Targets 2006, 7, 1011–1020. [Google Scholar] [CrossRef]
- Ramalho, R.; Almeida, J.; Beltrao, M.; Pirraco, A.; Costa, R.; Sokhatska, O.; Guardao, L.; Palmares, C.; Guimaraes, J.T.; Delgado, L.; et al. Neurogenic inflammation in allergen-challengedobese mice: A missing link in the obesity-asthma association? Exp. Lung Res. 2012, 38, 316–324. [Google Scholar] [CrossRef]
- Lavoie, K.L.; Bacon, S.L.; Labrecque, M.; Cartier, A.; Ditto, B. Higher BMI is associated with worse asthma control and quality of life but not asthma severity. Respir. Med. 2006, 100, 648–657. [Google Scholar] [CrossRef]
- Bluher, S.; Moschos, S.; Bullen, J., Jr.; Kokkotou, E.; Maratos-Flier, E.; Wiegand, S.J.; Sleeman, M.W.; Mantzoros, C.S. Ciliary neurotrophic factorAx15 alters energy homeostasis, decreases body weight, and improves metabolic control in diet-induced obese and UCP1-DTA mice. Diabetes 2004, 53, 2787–2796. [Google Scholar] [CrossRef]
- Ettinger, M.P.; Littlejohn, T.W.; Schwartz, S.L.; Weiss, S.R.; McIlwain, H.H.; Heymsfield, S.B.; Bray, G.A.; Roberts, W.G.; Heyman, E.R.; Stambler, N.; et al. Recombinant variant of ciliary neurotrophic factor for weight loss in obese adults: A randomized, dose-ranging study. JAMA 2003, 289, 1826–1832. [Google Scholar] [CrossRef]
- Chida, D.; Osaka, T.; Hashimoto, O.; Iwakura, Y. Combined interleukin-6 and interleukin-1 deficiency causes obesity in young mice. Diabetes 2006, 55, 971–977. [Google Scholar] [CrossRef]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef]
- Chawla, A.; Repa, J.J.; Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors and lipid physiology: Opening the X-files. Science 2001, 294, 1866–1870. [Google Scholar] [CrossRef]
- Lee, C.H.; Chawla, A.; Urbiztondo, N.; Liao, D.; Boisvert, W.A.; Evans, R.M.; Curtiss, L.K. Transcriptional repression of atherogenic inflammation: Modulation by PPARdelta. Science 2003, 302, 453–457. [Google Scholar] [CrossRef]
- Vats, D.; Mukundan, L.; Odegaard, J.I.; Zhang, L.; Smith, K.L.; Morel, C.R.; Wagner, R.A.; Greaves, D.R.; Murray, P.J.; Chawla, A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006, 4, 13–24. [Google Scholar] [CrossRef]
- Kubota, N.; Terauchi, Y.; Kubota, T.; Kumagai, H.; Itoh, S.; Satoh, H.; Yano, W.; Ogata, H.; Tokuyama, K.; Takamoto, I.; et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J. Biol. Chem. 2006, 281, 8748–8755. [Google Scholar] [CrossRef]
- Yamauchi, T.; Waki, H.; Kamon, J.; Murakami, K.; Motojima, K.; Komeda, K.; Miki, H.; Kubota, N.; Terauchi, Y.; Tsuchida, A.; et al. Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes. J. Clin. Investig. 2001, 108, 1001–1013. [Google Scholar]
- Jones, J.R.; Barrick, C.; Kim, K.A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6712. [Google Scholar] [CrossRef]
- Yamauchi, T.; Waki, H.; Kamon, J.; Murakami, K.; Motojima, K.; Komeda, K.; Miki, H.; Kubota, N.; Terauchi, Y.; Tsuchida, A.; et al. Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes. J. Clin. Investig. 2001, 108, 1001–1013. [Google Scholar]
- Luo, W.; Cao, J.; Li, J.; He, W. Adipose tissue-specific PPARgamma deficiency increases resistance to oxidative stress. Exp. Gerontol. 2008, 43, 154–163. [Google Scholar] [CrossRef]
- Bojic, L.A.; Sawyez, C.G.; Telford, D.E.; Edwards, J.Y.; Hegele, R.A.; Huff, M.W. Activation of peroxisome proliferator-activated receptor delta inhibits human macrophage foam cell formation and the inflammatory response induced by very low-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2919–2928. [Google Scholar] [CrossRef]
- Fruchart, J.C.; Duriez, P. Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today (Barc.) 2006, 42, 39–64. [Google Scholar] [CrossRef]
- Harano, Y.; Miyawaki, T.; Nabiki, J.; Shibachi, M.; Adachi, T.; Ikeda, M.; Ueda, F.; Nakano, T. Development of cookie test for the simultaneous determination of glucose intolerance, hyperinsulinemia, insulin resistance and postprandial dyslipidemia. Endocr. J. 2006, 53, 173–180. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Power of metabolomics in biomarker discovery and mining mechanisms of obesity. Obes. Rev. 2012, 14, 344–349. [Google Scholar] [CrossRef]
- Ament, Z.; Masoodi, M.; Griffin, J.L. Applications of metabolomics for understanding the action of peroxisome proliferator-activated receptors (PPARs) in diabetes, obesity and cancer. Genome Med. 2012, 4. [Google Scholar] [CrossRef]
- Graversen, J.H.; Madsen, M.; Moestrup, S.K. CD163: A signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int. J. Biochem. Cell Biol. 2002, 34, 309–314. [Google Scholar] [CrossRef]
- De Groot, D.M.; Vogel, G.; Dulos, J.; Teeuwen, L.; Stebbins, K.; Hamann, J.; Owens, B.M.; van Eenennaam, H.; Bos, E.; Boots, A.M. Therapeutic antibody targeting of CD97 in experimental arthritis: The role of antigen expression, shedding, and internalization on the pharmacokinetics of anti-CD97 monoclonal antibody 1B2. J. Immunol. 2009, 183, 4127–4134. [Google Scholar] [CrossRef]
- Altintas, M.M.; Azad, A.; Nayer, B.; Contreras, G.; Zaias, J.; Faul, C.; Reiser, J.; Nayer, A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J. Lipid Res. 2011, 52, 480–488. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Cochrane, D.E. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J. Neuroimmunol. 2004, 146, 1–12. [Google Scholar] [CrossRef]
- Vieira Dos Santos, R.; Magerl, M.; Martus, P.; Zuberbier, T.; Church, M.K.; Escribano, L.; Maurer, M. Topical sodium cromoglicate relieves allergen- and histamine-induced dermal pruritus. Br. J. Dermatol. 2010, 162, 674–676. [Google Scholar] [CrossRef]
- Okayama, Y.; Benyon, R.C.; Rees, P.H.; Lowman, M.A.; Hillier, K.; Church, M.K. Inhibition profiles of sodium cromoglycate and nedocromil sodium on mediator release from mast cells of human skin, lung, tonsil, adenoid and intestine. Clin. Exp. Allergy 1992, 22, 401–409. [Google Scholar] [CrossRef]
- Fox, C.C.; Wolf, E.J.; Kagey-Sobotka, A.; Lichtenstein, L.M. Comparison of human lung and intestinal mast cells. J. Allergy Clin. Immunol. 1988, 81, 89–94. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Periyalil, H.A.; Gibson, P.G.; Wood, L.G. Immunometabolism in Obese Asthmatics: Are We There Yet? Nutrients 2013, 5, 3506-3530. https://doi.org/10.3390/nu5093506
Periyalil HA, Gibson PG, Wood LG. Immunometabolism in Obese Asthmatics: Are We There Yet? Nutrients. 2013; 5(9):3506-3530. https://doi.org/10.3390/nu5093506
Chicago/Turabian StylePeriyalil, Hashim A., Peter G. Gibson, and Lisa G. Wood. 2013. "Immunometabolism in Obese Asthmatics: Are We There Yet?" Nutrients 5, no. 9: 3506-3530. https://doi.org/10.3390/nu5093506