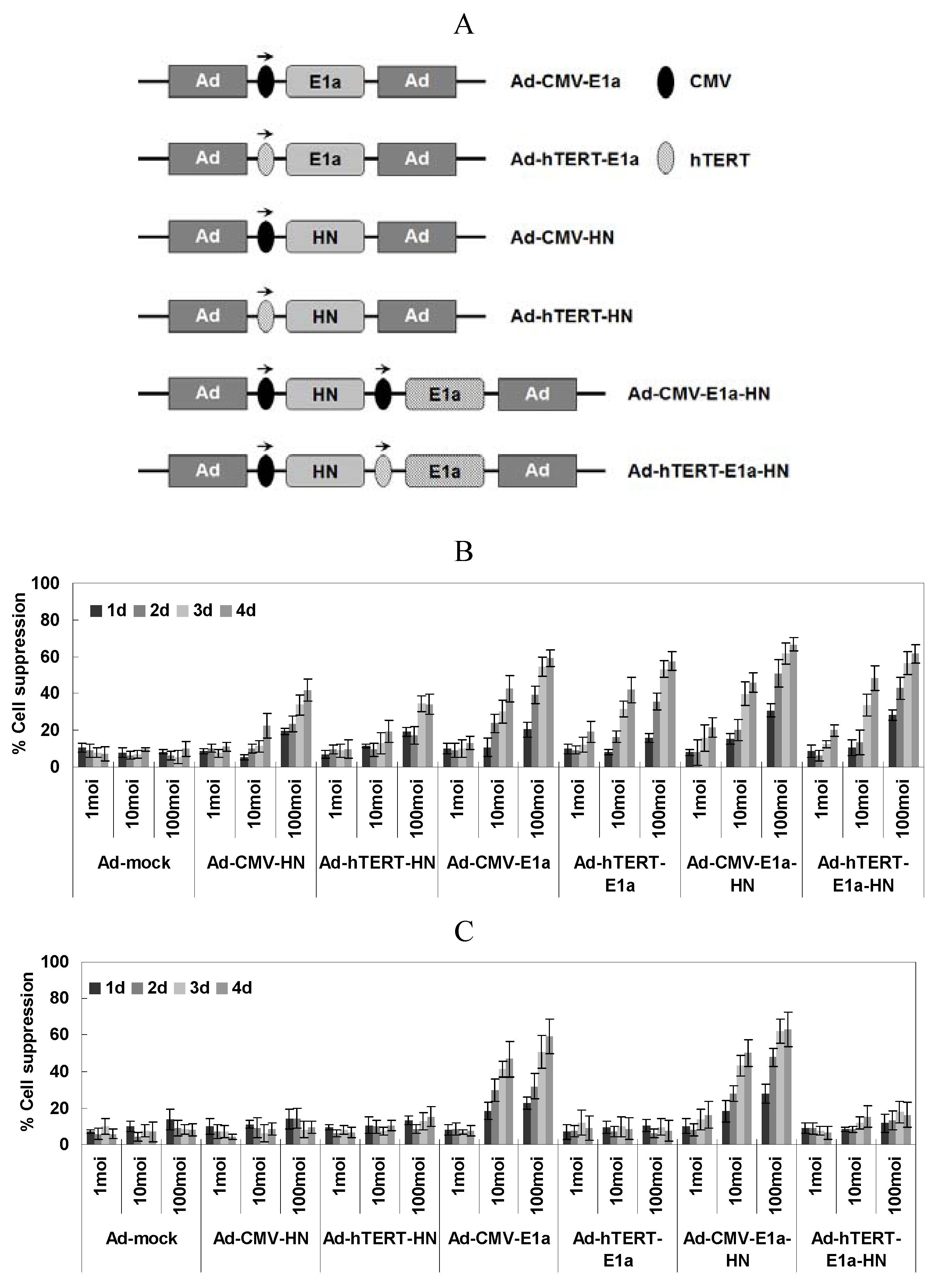
2.1. Specific Lethal Effects of Recombinant Adenoviruses in EC-109 Cells
With the aim of assessing cell viability of the recombinant adenovirus-infected EC-109 and normal human liver cell line L02, the MTT colorimetric assay was performed at various times post-infection and three different MOI of virus (
Figure 1B,C). In EC-109 cells (
Figure 1B), the suppression of cells by infection with replication-competent adenoviruses (Ad-CMV-E1a, Ad-hTERT-E1a, Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN) increased significantly over the infection time, especially in those infected with Ad-CMV-E1a-HN or Ad-hTERT-E1a-HN. Meanwhile, there was no significant difference between cells treated with Ad-CMV-HN or Ad-hTERT-HN. Furthermore, infection with Ad-CMV-E1a-HN, Ad-hTERT-E1a-HN, Ad-CMV-E1a or Ad-hTERT-E1a at an MOI of 10 or 100 obviously inhibited cell growth compared with treatment with the replication-incompetent adenoviruses after day 4. However, there was no significant difference in the growth of recombinant adenovirus cells infected with a MOI of 1. By contrast, the growth of L02 cells infected only with non-specific replication-competent adenoviruses (Ad-CMV-E1a or Ad-CMV-E1a-HN) was significantly inhibited at a MOI of 10 or 100 compared with infection at the MOI of 1 (
Figure 1C). Thus, replication-competent adenoviruses were much more effective than the replication-incompetent ones in suppressing EC-109 cells. Additionally, Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN could effectively restrain the growth of cultured EC-109 cells. Compared with the inhibitory effect of Ad-CMV-E1a-HN on L02 cells, Ad-hTERT-E1a-HN could replicate specifically in EC-109 cells and selectively restrict cell growth. Interestingly, the complex relationship between infection time and MOI was synergistic, and cell viability showed a non-rigorous dependence on both factors. Therefore, we performed the following experiments after infection at MOI of 100.
Figure 1.
(A) Schematic diagram of recombinant adenoviruses. Schematic of recombinant adenoviruses depicting the organizational elements. In non-specific replication-competent adenoviruses (Ad-CMV-E1a and Ad-CMV-E1a-HN), the CMV promoter drives E1a expression. hTERT and E1a genes were incorporated in the tumor-specific replication-competent adenovirus (Ad-hTERT-E1a). HN expression from the two replication-incompetent adenoviruses lacking the E1a gene, Ad-CMV-HN and Ad-hTERT-HN, is driven by CMV and hTERT promoters, respectively, and did not replicate in either cancer cells or normal cells. In the dual-specific anti-tumor recombinant adenovirus (Ad-hTERT-E1a-HN), which demonstrated both tumor-specific replication and cell growth inhibitory effects, the hTERT promoter drives E1a and the CMV promoter drives HN expression. (E1a, essential gene for adenovirus replication; HN, specific anti-tumor gene; CMV, cytomegalovirus promoter; hTERT: tumor-specific promoter.) Selective inhibitory effects of Ad-hTERT-E1a-HN were assessed on the human esophageal cancer EC-109 cells (B) and normal human liver L02 cells (C). Effects of different MOI and infection times on viability of EC-109 cells (B) and L02 cells (C). Cells were seeded in 96-well plates (1 × 104 cells/well) one day before infection with various concentrations (MOI 1, 10 and 100) of the indicated adenoviruses. Tumor viability was measured every day over a four-day period using the MTT colorimetric assay, and all measurements were performed in triplicate. Data are presented as means ± standard deviation (SD). In the EC-109 human esophageal cancer cells (B), Ad-CMV-E1a-HN, Ad-hTERT-E1a-HN, Ad-CMV-E1a and Ad-hTERT-E1a infection resulted in significant growth inhibition. In contrast, in L02 cells (C), only Ad-CMV-E1a or Ad-CMV-E1a-HN, but not Ad-CMV-HN, Ad-hTERT-HN, Ad-hTERT-E1a or Ad-hTERT-E1a-HN, inhibited cell growth.
Figure 1.
(A) Schematic diagram of recombinant adenoviruses. Schematic of recombinant adenoviruses depicting the organizational elements. In non-specific replication-competent adenoviruses (Ad-CMV-E1a and Ad-CMV-E1a-HN), the CMV promoter drives E1a expression. hTERT and E1a genes were incorporated in the tumor-specific replication-competent adenovirus (Ad-hTERT-E1a). HN expression from the two replication-incompetent adenoviruses lacking the E1a gene, Ad-CMV-HN and Ad-hTERT-HN, is driven by CMV and hTERT promoters, respectively, and did not replicate in either cancer cells or normal cells. In the dual-specific anti-tumor recombinant adenovirus (Ad-hTERT-E1a-HN), which demonstrated both tumor-specific replication and cell growth inhibitory effects, the hTERT promoter drives E1a and the CMV promoter drives HN expression. (E1a, essential gene for adenovirus replication; HN, specific anti-tumor gene; CMV, cytomegalovirus promoter; hTERT: tumor-specific promoter.) Selective inhibitory effects of Ad-hTERT-E1a-HN were assessed on the human esophageal cancer EC-109 cells (B) and normal human liver L02 cells (C). Effects of different MOI and infection times on viability of EC-109 cells (B) and L02 cells (C). Cells were seeded in 96-well plates (1 × 104 cells/well) one day before infection with various concentrations (MOI 1, 10 and 100) of the indicated adenoviruses. Tumor viability was measured every day over a four-day period using the MTT colorimetric assay, and all measurements were performed in triplicate. Data are presented as means ± standard deviation (SD). In the EC-109 human esophageal cancer cells (B), Ad-CMV-E1a-HN, Ad-hTERT-E1a-HN, Ad-CMV-E1a and Ad-hTERT-E1a infection resulted in significant growth inhibition. In contrast, in L02 cells (C), only Ad-CMV-E1a or Ad-CMV-E1a-HN, but not Ad-CMV-HN, Ad-hTERT-HN, Ad-hTERT-E1a or Ad-hTERT-E1a-HN, inhibited cell growth.
![Viruses 06 00856 g001]()
2.2. Effects of Recombinant Adenoviruses on EC-109 Cell Membrane
To analyze the mechanism of cell death, fluorescent assays were conducted after acridine orange (AO) and ethidium bromide (EB) staining to quantify and determine the relative percentages of live, apoptotic and necrotic EC-109 or L02 cells after infection with recombinant adenoviruses (
Figure 2A–C). Chromatin condensation and nuclear fragmentation are the hallmarks of apoptotic cells. The AO dye can permeate all cells and render the nuclei and cytoplasm green, while EB stains the nucleus red, dominating over the AO stain, only when the cell membrane is disrupted [
17]. As shown in
Figure 2A, all recombinant adenovirus-infected EC-109 cells presented bright green and orange staining, compared with the generally green signals of healthy control and Ad-mock-treated ones. Cytotoxicity was especially strong in the Ad-CMV-E1a-HN- and Ad-hTERT-E1a-HN-treated cells. In contrast, in L02 cells, only Ad-CMV-E1a- or Ad-CMV-E1a-HN-treated cells showed bright green and orange signals. With the AO/EB method, proportions of live, apoptotic and necrosis/late apoptotic cells after recombinant adenoviruses treatment could be quantified (
Figure 2B,C). The percentages of live cells in EC-109 cells infected with Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN were obviously lower than those observed with recombinant adenoviruses (
Figure 2B). Meanwhile, due to the loss of the tumor-specific effect of HN in L02 cells, only Ad-CMV-E1a- or Ad-CMV-E1a-HN-treated cells were cytotoxic and caused a higher proportion of apoptotic and/or necrotic cells (
Figure 2C). Although all of the recombinant adenoviruses restrained the growth of EC-109 cells via induction of apoptosis and necrosis, these results indicated that Ad-hTERT-E1a-HN had the strongest anti-tumor effect.
2.3. Expression of HN gene and Its Effect on Sialic Acid Content in vitro
The protein expression of the HN gene was observed on infected EC-109 and L02 cells by immunofluorescence analysis with the corresponding FITC-labeled antibody. As shown in
Figure 2D, the HN protein was expressed in L02 cells treated with Ad-CMV-HN, Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN; meanwhile, EC-109 cells infected with Ad-CMV-HN, Ad-hTERT-HN, Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN stained bright green, indicating the significant expression level of the HN gene, especially in Ad-hTERT-E1a-HN-treated cells. Furthermore, the total sialic acid content quantified using the 3,5-dihydroxytoluene method was compared between untreated cells and those of EC-109 or L02 cells treated with the various recombinant adenoviruses. In L02 cells (
Figure 2E), the sialic acid content of Ad-CMV-E1a- and Ad-CMV-E1a-HN-treated cells gradually decreased over the infection time, while cells infected with other recombinant adenoviruses retained significantly higher levels of sialic acid on the fourth day compared with those on the first day. In contrast, in EC-109 cells (
Figure 2F), at 24 h after infection with recombinant adenoviruses, no significant difference in the content of total sialic acid was found among the groups. Interestingly, at day 2 after infection, the total sialic acid levels of cells treated with Ad-CMV-HN, Ad-hTERT-HN, Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN began to decrease, while others groups showed a rising trend of the total sialic acid content. At a later time point (ay 3 post-infection), Ad-mock-infected and control groups contained significantly higher levels of sialic acid compared with the Ad-CMV-E1a and Ad-hTERT-E1a groups, but Ad-CMV-HN-, Ad-hTERT-HN-, Ad-CMV-E1a-HN- and Ad-hTERT-E1a-HN-treated cells had a notable decrease, especially the Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN groups. By day 4 post-infection, the sialic acid levels of the Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN treatment groups were almost eliminated. While the sialic acid levels in the Ad-CMV-HN and Ad-hTERT-HN groups decreased, they did not change significantly between the third and fourth day post-infection. However, the concentrations of sialic acid of the control group and the Ad-mock-, Ad-CMV-E1a- and Ad-hTERT-E1a-treated cells gradually increased, which was accompanied by cell proliferation. These results suggested that Ad-hTERT-E1a-HN could effectively decrease the sialic acid level in EC-109 cells and would be suitable as an anti-tumor treatment.
Figure 2.
Morphological changes of EC-109 and L02 cells infected with Ad-hTERT-E1a-HN by AO/EB staining. (A) Fluorescence images at 100× magnification show morphological changes of recombinant adenovirus-infected EC-109 and L02 cells stained with AO/EB. IPP analysis of EC-109 (B) and L02 cells (C) infected with recombinant adenovirus was carried out to quantify proportions of live, apoptotic and necrosis/late apoptotic populations. Microscopic images were captured and analyzed by the Image-Pro Plus software program. Data are means ± standard deviation (SD). L, normal cell; A, apoptotic cell; N/A, necrosis/late apoptotic cell. (D) Immunofluorescence detection of HN in EC109 and L02 cells with the corresponding FITC-labeled antibody. (E and F) Sialic acid levels in EC109 and L02 cells after treatment with recombinant adenoviruses. Sialic acid levels on EC109 and L02 cells infected at a MOI 10 of recombinant adenoviruses were measured every day over a four-day period using 3,5-dihydroxytoluene, and A560 values were detected with an ultraviolet spectrophotometer. The absorbance is directly proportional to the sialic acid concentration in the sample, which was calculated with the formula: experimental A560/standard A 560 × 1.94. Data are means ± SD.
Figure 2.
Morphological changes of EC-109 and L02 cells infected with Ad-hTERT-E1a-HN by AO/EB staining. (A) Fluorescence images at 100× magnification show morphological changes of recombinant adenovirus-infected EC-109 and L02 cells stained with AO/EB. IPP analysis of EC-109 (B) and L02 cells (C) infected with recombinant adenovirus was carried out to quantify proportions of live, apoptotic and necrosis/late apoptotic populations. Microscopic images were captured and analyzed by the Image-Pro Plus software program. Data are means ± standard deviation (SD). L, normal cell; A, apoptotic cell; N/A, necrosis/late apoptotic cell. (D) Immunofluorescence detection of HN in EC109 and L02 cells with the corresponding FITC-labeled antibody. (E and F) Sialic acid levels in EC109 and L02 cells after treatment with recombinant adenoviruses. Sialic acid levels on EC109 and L02 cells infected at a MOI 10 of recombinant adenoviruses were measured every day over a four-day period using 3,5-dihydroxytoluene, and A560 values were detected with an ultraviolet spectrophotometer. The absorbance is directly proportional to the sialic acid concentration in the sample, which was calculated with the formula: experimental A560/standard A 560 × 1.94. Data are means ± SD.
![Viruses 06 00856 g002]()
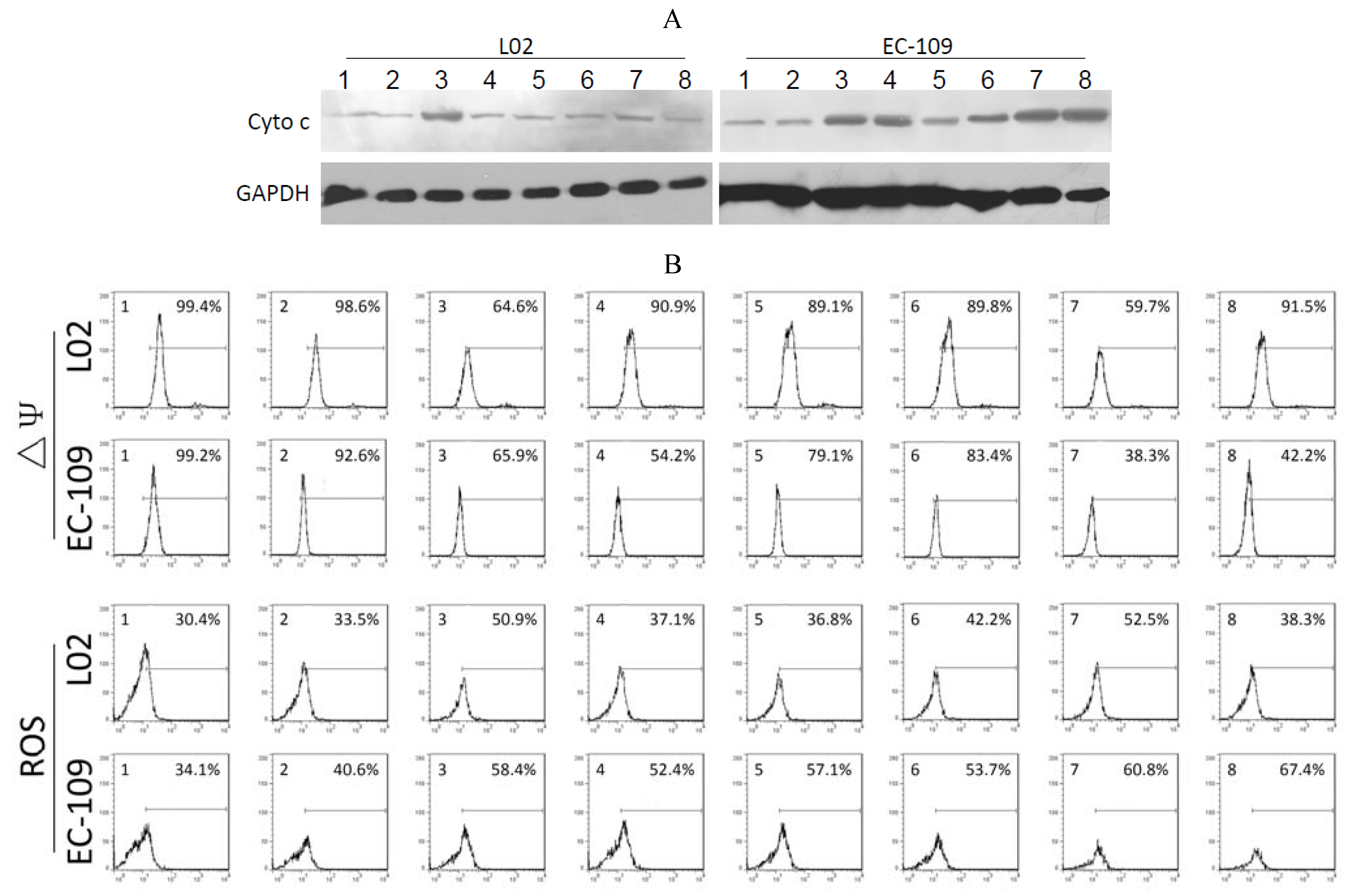
2.4. Effects of Recombinant Adenoviruses on Signaling Molecules in EC-109 Cells
As shown in
Figure 3A, significant quantities of cytochrome c were detected in the cytosol of Ad-CMV-E1a-, Ad-hTERT-E1a-, Ad-CMV-E1a-HN- and Ad-hTERT-E1a-HN-infected EC-109 cells. The levels of cytochrome c in cells treated with Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN were higher than in Ad-CMV-E1a- and Ad-hTERT-E1a-treated groups. However, only Ad-CMV-E1a- and Ad-CMV-E1a-HN-treated L02 cells showed a small amount of released cytochrome c, while other recombinant adenovirus had no significant effects in the L02 cells. As shown in
Figure 3B, significant ΔΨm losses were detected in Ad-CMV-E1a-HN- (38.3%) and Ad-hTERT-E1a-HN-infected (42.2%) EC-109 cells, but not in the cells treated with the other recombinant adenoviruses. The slight decrease in ΔΨm was also detected in Ad-CMV-E1a- (65.9%) and Ad-hTERT-E1a-infected (54.2%) cells. In L02 cells, only the Ad-CMV-E1a and Ad-CMV-E1a-HN treatment resulted in a ΔΨm loss of 64.6% and 59.7%, respectively, and the ΔΨm values of L02 cells treated with other recombinant adenovirus were similar to that of the untreated group.
The various recombinant adenoviruses showed differential abilities to elevate levels of ROS (
Figure 3B). EC-109 cells infected with Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN showed a significant increase in ROS of 60.8% and 67.4%, respectively, while in recombinant adenovirus-treated L02 cells, only Ad-CMV-E1a and Ad-CMV-E1a-HN could raise the levels of ROS by 50.9% and 52.5%, respectively. These experiments indicated that the apoptotic pathway in EC-109 cells triggered by Ad-hTERT-E1a-HN was associated with the release of cytochrome c, loss of ΔΨm and increase of ROS. Compared with other recombinant adenovirus, Ad-hTERT-E1a-HN showed a distinct level of tumor-specific targeting and killing.
Figure 3.
Analysis of mitochondrial permeability transition of the recombinant adenovirus-treated EC-109 cells and L02 cells. (A) Expression of cytochrome c in recombinant adenovirus-treated EC-109 and L02 cells was detected by Western blot. (B) Flow cytometric determination of ΔΨm and ROS. The proportions were calculated as follows: number of stained cells/total number of cells (%). 1. Control; 2. Ad-mock; 3. Ad-CMV-E1a; 4. Ad-hTERT-E1a; 5. Ad-CMV-HN; 6. Ad-hTERT-HN; 7. Ad-CMV-E1a-HN; 8. Ad-hTERT-E1a-HN.
Figure 3.
Analysis of mitochondrial permeability transition of the recombinant adenovirus-treated EC-109 cells and L02 cells. (A) Expression of cytochrome c in recombinant adenovirus-treated EC-109 and L02 cells was detected by Western blot. (B) Flow cytometric determination of ΔΨm and ROS. The proportions were calculated as follows: number of stained cells/total number of cells (%). 1. Control; 2. Ad-mock; 3. Ad-CMV-E1a; 4. Ad-hTERT-E1a; 5. Ad-CMV-HN; 6. Ad-hTERT-HN; 7. Ad-CMV-E1a-HN; 8. Ad-hTERT-E1a-HN.
2.5. Effects of Recombinant Adenoviruses on EC-109 Cell-Derived Tumors in Vivo
To evaluate the anti-tumor potential of the vectors
in vivo, we treated tumor-bearing mice with recombinant adenoviruses via intratumoral or intravenous injections. Each tumor was measured twice a week, and all animals in the study were sacrificed after nine weeks. The growth kinetics of the tumors treated with intratumoral injections are shown in
Figure 4A. Compared with the saline control, Ad-mock- and Ad-CMV-HN-infected groups,other groups infected with recombinant adenoviruses showed suppression of tumor growth. Treatment of mice with Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN induced a potent anti-tumor response, especially in the Ad-hTERT-E1a-HN-infected group, in which the tumor volume was significantly reduced and close to regression. By contrast, the Ad-hTERT-HN-, Ad-CMV-E1a- and Ad-hTERT-E1a-infected tumors slowly grew and gradually resumed their growth at six weeks. In mice injected via the intravenous route (
Figure 4B), tumors of the Ad-CMV-E1a-HN- and Ad-hTERT-E1a-HN-infected groups decreased most significantly in size, compared with the saline control, Ad-mock- and Ad-mock-infected groups. Growth of the Ad-CMV-E1a-, Ad-hTERT-E1a-, Ad-CMV-HN- and Ad-hTERT-HN-treated tumors were also suppressed, compared with saline-treated and Ad-mock-infected groups; however, there was no significant difference in growth rates of the tumors in these groups. In the intratumorally injected groups, the mean tumor volumes of the recombinant adenovirus treatment groups were reduced obviously and were basically always maintained at a lower level, compared with saline-treated and Ad-mock-infected groups (
Figure 4C). Notably, the tumors nearly disappeared in the Ad-hTERT-E1a-HN-infected group. The results from
Figure 4A,C indicated that the intratumoral injection of Ad-CMV-E1a-HN or Ad-hTERT-E1a-HN could reduce tumor volume efficiently.
Mean tumor volumes in the intravenously injected groups were also evaluated (
Figure 4D). Although the tumors infected with Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN gradually resumed their growth after two weeks, their tumor volumes were the lowest compared with other groups. The tumor volumes of the Ad-CMV-E1a-, Ad-hTERT-E1a-, Ad-CMV-HN- and Ad-hTERT-HN-infected groups were reduced compared with those of the saline-treated and Ad-mock-infected groups, while there were no significant differences between the groups. As shown in
Figure 4B,D, the efficacy in intratumorally injected groups was better than that of the intravenously injected groups. The main reason for this difference may be that the adenoviruses delivered by direct intratumoral injection could produce a more rapid effect than those by intravenous injection. Ad-CMV-E1a-HN- and Ad-hTERT-E1a-HN-treated mice could reduce tumor burdens more significantly than other recombinant adenoviruses.
We also evaluated the lifespan of mice following treatment. As shown in
Figure 4E, when the injections were performed intratumorally, infection with Ad-CMV-E1a, Ad-hTERT-E1a, Ad-CMV-E1a-HN or Ad-hTERT-E1a-HN significantly improved the mean survival time, while the saline-treated and Ad-mock-, Ad-CMV-HN- and Ad-hTERT-HN-infected groups had lower mean survival times of 48.5 days, 43.5 days, 59.5 days and 55.5 days, respectively. These results demonstrate that intratumoral injection of replication-competent adenoviruses could improve survival effectively in mice.
In the intravenously injected groups, Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN could increase survival rates of nude mice to the mean survival time of 63 days, which was significantly better than that observed in Ad-CMV-HN-, Ad-hTERT-HN-, Ad-CMV-E1a- and Ad-hTERT-E1a-infected groups (
Figure 4F). As expected, saline-treated and Ad-mock-infected mice had the worst survival rates with mean survival times of only 39.5 days and 43.25 days, respectively. The results indicate that intravenous injections of Ad-CMV-E1a-HN and Ad-hTERT-E1a-HN conferred significant survival benefits
in vivo.
Figure 4.
Effects of recombinant adenovirus on tumors established by xenografting EC-109 cells in BALB/c nude mice. (A) Tumor growth kinetics of mice that received intratumoral injections of adenovirus; (B) Tumor growth kinetics of mice that received intravenous injections adenovirus; (C) Mean tumor volumes in intratumorally injected groups; (D) Mean tumor volumes in intravenous injections groups; (E) Survival curve of mice treated intratumorally; (F) Survival curve of mice treated intravenously. The day of the first injection was considered day 0. Data are presented as means ± SD.
Figure 4.
Effects of recombinant adenovirus on tumors established by xenografting EC-109 cells in BALB/c nude mice. (A) Tumor growth kinetics of mice that received intratumoral injections of adenovirus; (B) Tumor growth kinetics of mice that received intravenous injections adenovirus; (C) Mean tumor volumes in intratumorally injected groups; (D) Mean tumor volumes in intravenous injections groups; (E) Survival curve of mice treated intratumorally; (F) Survival curve of mice treated intravenously. The day of the first injection was considered day 0. Data are presented as means ± SD.
2.6. Discussion
Cancer is a major cause of death worldwide. Although significantly improved over recent decades, the efficacy of tumor treatments is still limited. Thus, developing novel therapeutic strategies for cancer patients remains a constant need. Oncolytic adenoviruses are promising tools in cancer therapeutics due to their ability to be genetically manipulated and exhibit multiple distinct anti-cancer mechanisms, including direct lysis, apoptosis induction, expression of toxic proteins, autophagy and shutting-down of protein synthesis, as well as the induction of anti-tumoral immunity [
5]. Assessing various therapeutic genes to insert into the viral genome has been a major focus in cancer virotherapy, and types of transgenes thus far considered for this purpose include tumor suppressor, pro-apoptotic, anti-angiogenic, “suicide” and immunomodulatory genes [
5]. Xiao-Ping He
et al. [
18] previously showed that the anti-tumor effect of a conditionally replicating adenovirus (CRAd) vector modified by incorporation of an anti-angiogenesis inhibitor gene (CRAd-Cans) was even more potent than that of the replication-deficient adenovirus Ad5-Cans against pancreatic cancer both
in vivo and
in vitro. Ji
et al. [
19] suggested that hTERT promoter-driven oncolytic CRAd vector in combination with HSV tk /GCV gene therapy could effectively reduce growth of human retinoblastoma in an orthotopic nude mouse model but not in primary human retinal pigment epithelial cells (hRPE). Lin Fang
et al. [
20] inserted a novel 720-bp truncated minimal E1a gene (mE1a) and hTERT into an oncolytic adenoviral vector lacking the E1b gene. The constructed vector was shown to infect and replicate selectively with high efficiency and exerted an effective anti-tumor activity in human cancer cell lines as well as in hepatocarcinoma (HepG II) xenografted nude BALB/c mice [
20].
In the present study, we constructed a novel dual specific anti-tumor oncolytic adenovirus Ad-hTERT-E1a-HN by inserting NDV HN gene and hTERT promoter into a RAPAd.I adenovirus vector, as well as the control recombinant adenoviruses (
Figure 1A). Furthermore, we evaluated the anti-tumor effects of these novel oncolytic viruses on esophageal cancer
in vitro and
in vivo. In order to demonstrate that Ad-hTERT-E1a-HN selectively replicated in human EC-109 tumor cells, but not in normal cells, we also used Ad-hTERT-E1a-HN to infect the human L02 cells. With the extension of infection times and the increase of the infective dose (a MOI of 1, 10 or 100), the inhibitory effects on EC-109 cells treated with Ad-CMV-E1a-HN and Ad-hTERTE1a-HN became more obvious than those with other recombinant adenoviruses. In L02 cells, the inhibition was still obvious in Ad-CMV-E1a-HN-treated cells, compared with the slight inhibitory effect in the Ad-hTERT-E1a-HN-treated group. The results showed that Ad-hTERT-E1a-HN could replicate and restrict growth specifically in EC-109 cells (
Figure 1B,C). Furthermore, cell viability showed a non-rigorous dependence on both infection time and MOI.
Apoptosis is a cell death program that eliminates harmful and severely damaged cells and maintains tissue homeostasis in multicellular organisms [
21]. A number of viruses have the ability to induce apoptosis and contribute to cytopathic effects in infected cells [
22]. NDV is a tumor selective and intrinsically oncolytic avian paramyxovirus that has been considered as a potentially powerful tool for cancer therapy [
23]. Several studies have shown that NDV can induce apoptosis in various cancer cell types by activating the mitochondrial death pathway (intrinsic pathway) and the death receptor pathway (extrinsic pathway) [
24,
25,
26], ultimately inducing caspase-dependent pathways in infected cells that lead to the biochemical and morphological changes characteristic of apoptosis [
22,
27]. To avoid the side effect after treatment with the virus alone, we were interested in studying the anti-tumor mechanism of apoptosis induction by HN. In the present study, we primarily investigated the effects of Ad-hTERT-E1a-HN on cell surface sialic acid levels, which are associated with tumor cell behavior, such as invasiveness and metastasis. The results showed that Ad-hTERT-E1a-HN could effectively decrease the sialic acid level on EC-109 cells, while it did not affect the total sialic acid content in L02 cells. The AO/EB staining assay used in this study to analyze cell death pathways could quantify the relative proportions of live, apoptotic and necrotic recombinant adenoviruses-infected cells (
Figure 2A–C). The results indicated that Ad-hTERT-E1a-HN could significantly restrain the growth of EC-109 cells via induction of apoptosis and necrosis, while it did not affect the normal L02 cells. Furthermore, Ad-hTERT-E1a-HN was found to cause the apparent increase of reactive oxygen species (ROS), and also significantly reduce the ΔΨm and release of cytochrome c in EC-109 cells. By contrast, these effects of Ad-hTERT-E1a-HN on L02 cells were minor. Therefore, compared with Ad-CMV-E1a-HN and other recombinant adenoviruses, Ad-hTERT-E1a-HN had the potential ability to specifically kill tumor cells by inducing the apoptosis pathway.
Anti-tumor activities of the recombinant adenoviruses were also evaluated in a human esophageal cancer xenograft mouse model, which further confirmed the efficacies observed
in vitro. Esophageal cancer is considered one of the most refractory pernicious malignant tumors with a propensity for local progression and distant dissemination. Despite ongoing research in the treatment of esophageal cancers, the prognosis for long-term survival remains poor. A five-year overall survival for patients undergoing resection for esophageal cancer was reported to be between 25% and 39%, depending on surgical modality and the use of neoadjuvant therapy [
28]. In order to improve the poor prognosis, many strategies have been implemented. Ye
et al. [
29] used curcumin, (-)-epigallocatechin-3-gallate (EGCG), lovastatin and their combinations to treat esophageal cancer TE-8 and SKGT-4 cells. Although all of these treatments were found to significantly reduce the viability and invasion capacity of esophageal cancer cells
in vitro, they were much less effective when tested
in vivo in nude mouse xenografts, especially curcumin or lovastatin used individually [
30]. Papineni
et al. [
31] also examined the effects of daily administration of tolfenamic acid (TA, 20 mg/kg/day) on tumor growth in athymic nude mice bearing SEC-1 cells as xenografts. Although the results showed that this dose of TA could significantly inhibit tumor growth and tumor weight, at the same time it increased apoptosis and decreased Sp1 and c-Met staining in tumors from treated mice; however, TA did not achieve a complete response
in vivo using an animal model of esophageal cancer [
31]. In our study, effects of various recombinant adenoviruses were evaluated via intratumoral (A) and intravenous (B) injections into BALB/c nude mice xenografted with esophageal cancer EC-109 cells. Ad-hTERT-E1a-HN was found to have significant anti-tumor effects compared with those of other recombinant adenoviruses. Injection of Ad-hTERT-E1a-HN directly into tumors resulted in a complete response to treatment (
Figure 4A,C) and the longest mean survival time of 63 days, which were the most optimal outcomes compared with results from other recombinant adenovirus-treated groups (
Figure 4E). When infection was carried out intravenously, Ad-hTERT-E1a-HN did not lead to complete tumor regression, although it could still suppress the growth of the tumor (
Figure 4B,D) and significantly increase the survival rate of nude mice (
Figure 4F), compared with other groups. We demonstrated that the efficacy of intratumoral injection was greater than that of intravenous injection, primarily due to the direct deposition of the adenovirus at the tumor site which facilitated infection and rapid induction of anti-tumor effects. Furthermore, we did not observe any toxic effects after injection of Ad-hTERT-E1a-HN during the
in vivo experiments described here. These encouraging results showing the curative effectiveness of Ad-hTERT-E1a-HN indicated that it will be a potentially highly potent, effective and safe anticancer agent.