The Evolution of Adenoviral Vectors through Genetic and Chemical Surface Modifications
Abstract
:1. Introduction
Immunology of Ad Vectors
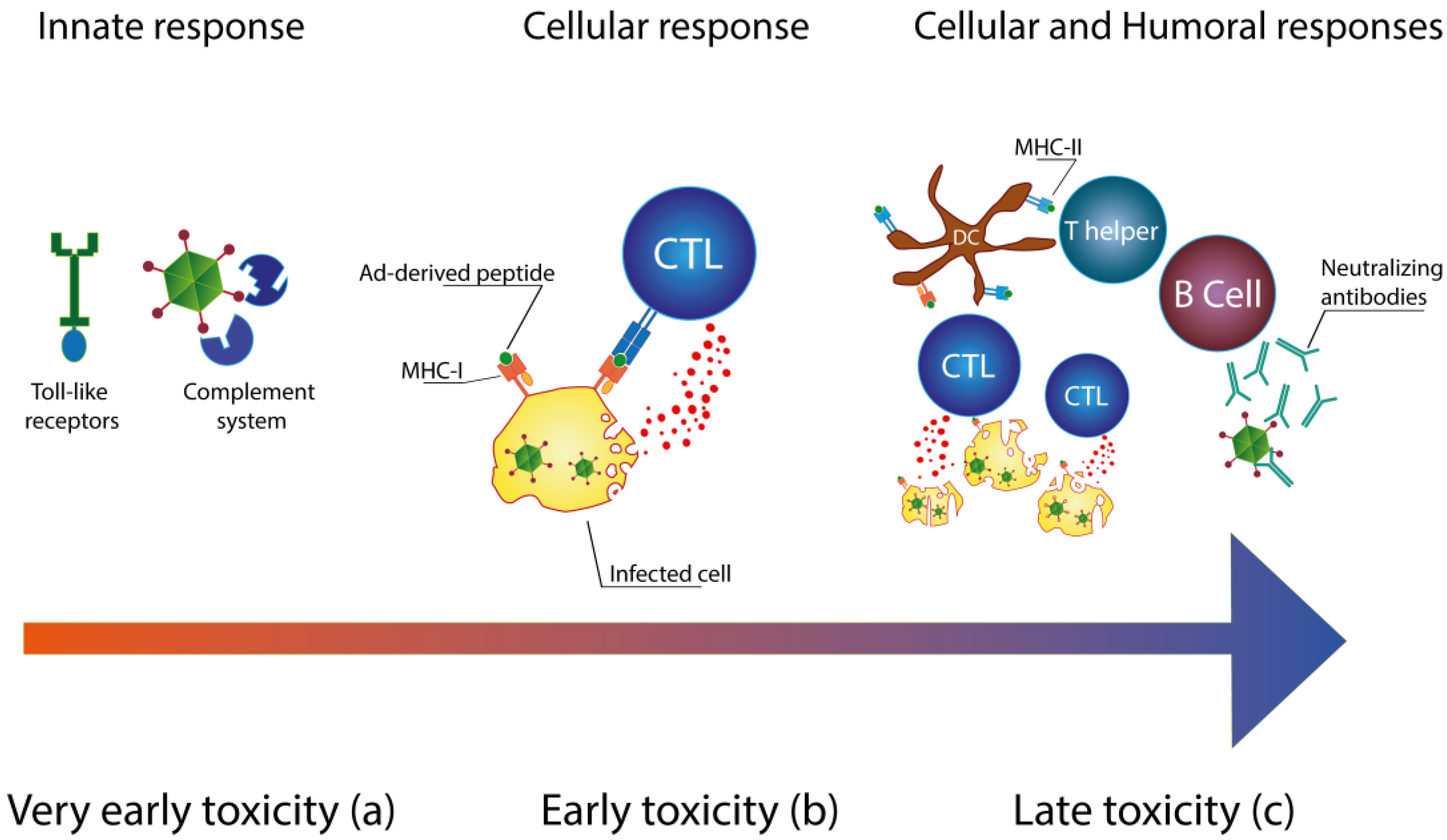
2. Reducing the Immunogenicity of Ad Vectors
2.1. Genetic Modifications
2.2. Improving the Safety Profile by Chemical and Non-Chemical Modifications
2.2.1. PEGylation as a Tool to Reduce the Immunogenicity
2.2.2. New Generation of Biomaterials for Less Immunogenic Ad Vectors
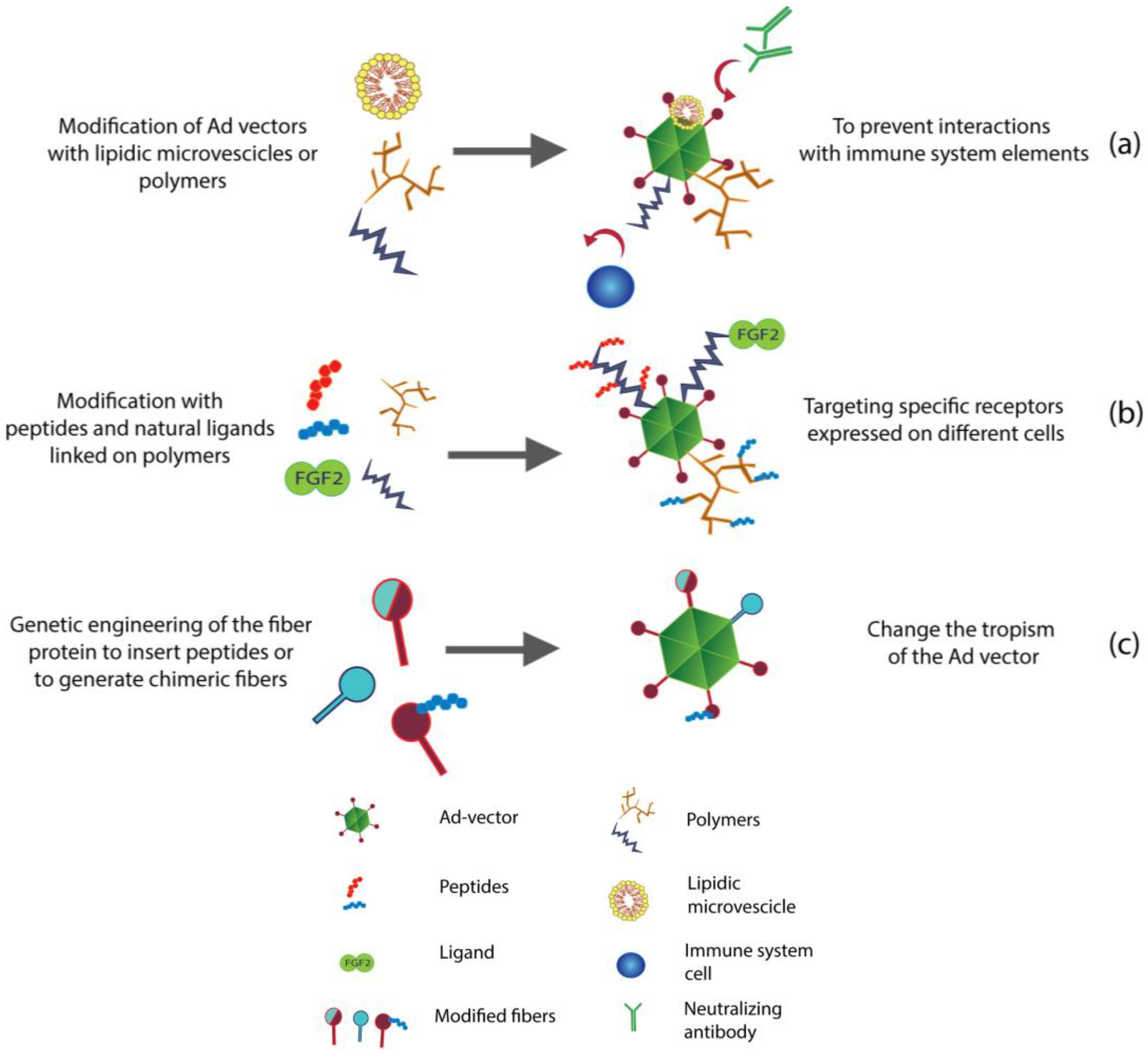
3. Re-Targeting of Ad Vectors
3.1. Genetic Engineering to Re-Target Ad Vectors
3.1.1. Use of Chimeric Fibers to Re-Target Ad
3.1.2. Insertion of Peptides and Other Ligands in the Structure of Ad Vectors
3.2. Chemical and Non-Chemical Modifications to Re-Target Ad Vectors
3.2.1. Lipid Envelopes for Liver De-Targeting
3.2.2. PEGylation as a Liver De-Targeting Tool
3.2.3. Liver De-Targeting by New Generation of Polymers
3.2.4. Re-Targeting with Microbeads or Magnetic Particles (Mags)
3.2.5. Accurate Re-Targeting of Ad Vectors with Peptide Motifs and Ligands
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Rosenberg, S.A.; Aebersold, P.; Cornetta, K.; Kasid, A.; Morgan, R.A.; Moen, R.; Karson, E.M.; Lotze, M.T.; Yang, J.C.; Topalian, S.L.; et al. Gene transfer into humans—Immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med. 1990, 323, 570–578. [Google Scholar] [CrossRef]
- Raper, S.E.; Chirmule, N.; Lee, F.S.; Wivel, N.A.; Bagg, A.; Gao, G.-P.; Wilson, J.M.; Batshaw, M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003, 80, 148–158. [Google Scholar] [CrossRef]
- Ginn, S.L.; Alexander, I.E.; Edelstein, M.L.; Abedi, M.R.; Wixon, J. Gene therapy clinical trials worldwide to 2012—An update. J. Gene Med. 2013, 15, 65–77. [Google Scholar] [CrossRef]
- Liu, Q.; Muruve, D.A. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003, 10, 935–940. [Google Scholar] [CrossRef]
- Croyle, M.A.; Le, H.T.; Linse, K.D.; Cerullo, V.; Toietta, G.; Beaudet, A.; Pastore, L. Pegylated helper-dependent adenoviral vectors: Highly efficient vectors with an enhanced safety profile. Gene Ther. 2005, 12, 579–587. [Google Scholar] [CrossRef]
- Cerullo, V.; Seiler, M.P.; Mane, V.; Brunetti-Pierri, N.; Clarke, C.; Bertin, T.K.; Rodgers, J.R.; Lee, B. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 2007, 15, 378–385. [Google Scholar] [CrossRef]
- Hartman, Z.C.; Kiang, A.; Everett, R.S.; Serra, D.; Yang, X.Y.; Clay, T.M.; Amalfitano, A. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol. 2007, 81, 1796–1812. [Google Scholar] [CrossRef]
- Appledorn, D.M.; McBride, A.; Seregin, S.; Scott, J.M.; Schuldt, N.; Kiang, A.; Godbehere, S.; Amalfitano, A. Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther. 2008, 15, 1606–1617. [Google Scholar]
- Jiang, H.; Wang, Z.; Serra, D.; Frank, M.M.; Amalfitano, A. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol. Ther. 2004, 10, 1140–1142. [Google Scholar] [CrossRef]
- Kiang, A.; Hartman, Z.C.; Everett, R.S.; Serra, D.; Jiang, H.; Frank, M.M.; Amalfitano, A. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol. Ther. 2006, 14, 588–598. [Google Scholar] [CrossRef]
- Rhee, E.G.; Blattman, J.N.; Kasturi, S.P.; Kelley, R.P.; Kaufman, D.R.; Lynch, D.M.; la Porte, A.; Simmons, N.L.; Clark, S.L.; Pulendran, B.; et al. Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J. Virol. 2011, 85, 315–323. [Google Scholar] [CrossRef]
- Hartman, Z.C.; Black, E.P.; Amalfitano, A. Adenoviral infection induces a multi-faceted innate cellular immune response that is mediated by the toll-like receptor pathway in A549 cells. Virology 2007, 358, 357–372. [Google Scholar] [CrossRef]
- Muruve, D.A.; Barnes, M.J.; Stillman, I.E.; Libermann, T.A. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 1999, 10, 965–976. [Google Scholar] [CrossRef]
- Seregin, S.S.; Aldhamen, Y.A.; Appledorn, D.M.; Hartman, Z.C.; Schuldt, N.J.; Scott, J.; Godbehere, S.; Jiang, H.; Frank, M.M.; Amalfitano, A. Adenovirus capsid-display of the retro-oriented human complement inhibitor daf reduces Ad vector-triggered immune responses in vitro and in vivo. Blood 2010, 116, 1669–1677. [Google Scholar] [CrossRef]
- Lieber, A.; He, C.Y.; Meuse, L.; Schowalter, D.; Kirillova, I.; Winther, B.; Kay, M.A. The role of kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 1997, 71, 8798–8807. [Google Scholar]
- Piccolo, P.; Vetrini, F.; Mithbaokar, P.; Grove, N.C.; Bertin, T.; Palmer, D.; Ng, P.; Brunetti-Pierri, N. SR-A and SREC-I are kupffer and endothelial cell receptors for helper-dependent adenoviral vectors. Mol. Ther. 2013, 21, 767–774. [Google Scholar] [CrossRef]
- Nazir, S.A.; Metcalf, J.P. Innate immune response to adenovirus. J. Invest. Med. 2005, 53, 292–304. [Google Scholar] [CrossRef]
- Muruve, D.A. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004, 15, 1157–1166. [Google Scholar] [CrossRef]
- Brunetti-Pierri, N.; Palmer, D.J.; Beaudet, A.L.; Carey, K.D.; Finegold, M.; Ng, P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 2004, 15, 35–46. [Google Scholar] [CrossRef]
- Yang, Y.; Su, Q.; Wilson, J.M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J. Virol. 1996, 70, 7209–7212. [Google Scholar]
- Yang, Y.; Ertl, H.C.; Wilson, J.M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1994, 1, 433–442. [Google Scholar] [CrossRef]
- Yang, Y.; Wilson, J.M. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J. Immunol. 1995, 155, 2564–2570. [Google Scholar]
- Yang, Y.; Greenough, K.; Wilson, J.M. Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver. Gene Ther. 1996, 3, 412–420. [Google Scholar]
- Yang, Y.; Jooss, K.U.; Su, Q.; Ertl, H.C.; Wilson, J.M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996, 3, 137–144. [Google Scholar]
- Seiler, M.P.; Cerullo, V.; Lee, B. Immune response to helper dependent adenoviral mediated liver gene therapy: Challenges and prospects. Curr. Gene Ther. 2007, 7, 297–305. [Google Scholar] [CrossRef]
- Thomas, G.P.; Mathews, M.B. DNA replication and the early to late transition in Adenovirus infection. Cell 1980, 22, 523–533. [Google Scholar] [CrossRef]
- Steegenga, W.T.; Shvarts, A.; Riteco, N.; Bos, J.L.; Jochemsen, A.G. Distinct regulation of p53 and p73 activity by adenovirus E1A, E1B, and E4orf6 proteins. Mol. Cell. Biol. 1999, 19, 3885–3894. [Google Scholar]
- Zamanian, M.; la Thangue, N.B. Adenovirus E1A prevents the retinoblastoma gene product from repressing the activity of a cellular transcription factor. EMBO J. 1992, 11, 2603–2610. [Google Scholar]
- Kelly, T.J., Jr.; Lewis, A.M., Jr. Use of nondefective adenovirus-simian virus 40 hybrids for mapping the simian virus 40 genome. J. Virol. 1973, 12, 643–652. [Google Scholar]
- Fu, J.; Li, L.; Bouvier, M. Adenovirus E3–19K proteins of different serotypes and subgroups have similar, yet distinct, immunomodulatory functions toward major histocompatibility class I molecules. J. Biol. Chem. 2011, 286, 17631–17639. [Google Scholar] [CrossRef]
- Schowalter, D.B.; Tubb, J.C.; Liu, M.; Wilson, C.B.; Kay, M.A. Heterologous expression of adenovirus E3-GP19K in an E1A-deleted adenovirus vector inhibits MHC I expression in vitro, but does not prolong transgene expression in vivo. Gene Ther. 1997, 4, 351–360. [Google Scholar]
- Flomenberg, P.; Gutierrez, E.; Hogan, K.T. Identification of class I mhc regions which bind to the adenovirus E3-19K protein. Mol. Immunol. 1994, 31, 1277–1284. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Black, H.B.; Goldwasser, E.; Leiden, J.M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat. Med. 1996, 2, 545–550. [Google Scholar] [CrossRef]
- Wohlleber, D.; Kashkar, H.; Gartner, K.; Frings, M.K.; Odenthal, M.; Hegenbarth, S.; Borner, C.; Arnold, B.; Hammerling, G.; Nieswandt, B.; et al. Tnf-induced target cell killing by ctl activated through cross-presentation. Cell Rep. 2012, 2, 478–487. [Google Scholar] [CrossRef]
- Silva, A.C.; Peixoto, C.; Lucas, T.; Kuppers, C.; Cruz, P.E.; Alves, P.M.; Kochanek, S. Adenovirus vector production and purification. Curr. Gene Ther. 2010, 10, 437–455. [Google Scholar] [CrossRef]
- Janssen, J.M.; Liu, J.; Skokan, J.; Goncalves, M.A.; de Vries, A.A. Development of an adeasy-based system to produce first- and second-generation adenoviral vectors with tropism for car- or cd46-positive cells. J. Gene Med. 2013, 15, 1–11. [Google Scholar]
- Brunetti-Pierri, N.; Ng, P. Helper-dependent adenoviral vectors for liver-directed gene therapy. Hum. Mol. Genet. 2011, 20, R7–R13. [Google Scholar] [CrossRef]
- Toietta, G.; Pastore, L.; Cerullo, V.; Finegold, M.; Beaudet, A.L.; Lee, B. Generation of helper-dependent adenoviral vectors by homologous recombination. Mol. Ther. 2002, 5, 204–210. [Google Scholar] [CrossRef]
- Palmer, D.J.; Ng, P. Characterization of helper-dependent adenoviral vectors. Cold Spring Harbor Protoc. 2011, 2011, 867–870. [Google Scholar]
- Muruve, D.A.; Cotter, M.J.; Zaiss, A.K.; White, L.R.; Liu, Q.; Chan, T.; Clark, S.A.; Ross, P.J.; Meulenbroek, R.A.; Maelandsmo, G.M.; et al. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J. Virol. 2004, 78, 5966–5972. [Google Scholar] [CrossRef]
- Vetrini, F.; Ng, P. Gene Therepy with helper-dependent adenoviral vectors: Current advances and future perspectives. Viruses 2010, 2, 1886–1917. [Google Scholar] [CrossRef]
- Coughlan, L.; Vallath, S.; Gros, A.; Gimenez-Alejandre, M.; van Rooijen, N.; Thomas, G.J.; Baker, A.H.; Cascallo, M.; Alemany, R.; Hart, I.R. Combined fiber modifications both to target αvβ6 and detarget the coxsackievirus-adenovirus receptor improve virus toxicity profiles in vivo but fail to improve antitumoral efficacy relative to adenovirus serotype 5. Hum. Gene Ther. 2012, 23, 960–979. [Google Scholar] [CrossRef]
- Hiwasa, K.; Nagaya, H.; Terao, S.; Acharya, B.; Hamada, K.; Mizuguchi, H.; Gotoh, A. Improved gene transfer into bladder cancer cells using adenovirus vector containing RGD motif. Anticancer Res. 2012, 32, 3137–3140. [Google Scholar]
- Wonganan, P.; Croyle, M.A. Pegylated adenoviruses: From mice to monkeys. Viruses 2010, 2, 468–502. [Google Scholar] [CrossRef]
- Wonganan, P.; Clemens, C.C.; Brasky, K.; Pastore, L.; Croyle, M.A. Species differences in the pharmacology and toxicology of pegylated helper-dependent adenovirus. Mol. Pharm. 2011, 8, 78–92. [Google Scholar] [CrossRef]
- Leggiero, E.; Astone, D.; Cerullo, V.; Lombardo, B.; Mazzaccara, C.; Labruna, G.; Sacchetti, L.; Salvatore, F.; Croyle, M.; Pastore, L. PEGylated helper-dependent adenoviral vector expressing human Apo A-I for gene therapy in LDLR-deficient mice. Gene Ther. 2013, 20, 1124–1130. [Google Scholar] [CrossRef] [Green Version]
- Kreppel, F.; Kochanek, S. Modification of adenovirus gene transfer vectors with synthetic polymers: A scientific review and technical guide. Mol. Ther. 2008, 16, 16–29. [Google Scholar] [CrossRef]
- Eto, Y.; Yoshioka, Y.; Ishida, T.; Yao, X.; Morishige, T.; Narimatsu, S.; Mizuguchi, H.; Mukai, Y.; Okada, N.; Kiwada, H.; et al. Optimized pegylated adenovirus vector reduces the anti-vector humoral immune response against adenovirus and induces a therapeutic effect against metastatic lung cancer. Biol. Pharm. Bull. 2010, 33, 1540–1544. [Google Scholar] [CrossRef]
- Ahn, C.H.; Chae, S.Y.; Bae, Y.H.; Kim, S.W. Synthesis of biodegradable multi-block copolymers of poly(l-lysine) and poly(ethylene glycol) as a non-viral gene carrier. J. Control. Release 2004, 97, 567–574. [Google Scholar]
- Zeng, Q.; Han, J.; Zhao, D.; Gong, T.; Zhang, Z.; Sun, X. Protection of adenovirus from neutralizing antibody by cationic peg derivative ionically linked to adenovirus. Int. J. Nanomedicine 2012, 7, 985–997. [Google Scholar]
- Fisher, K.D.; Seymour, L.W. HPMA copolymers for masking and retargeting of therapeutic viruses. Adv. Drug Delivery Rev. 2010, 62, 240–245. [Google Scholar] [CrossRef]
- Wang, C.H.; Chan, L.W.; Johnson, R.N.; Chu, D.S.; Shi, J.; Schellinger, J.G.; Lieber, A.; Pun, S.H. The transduction of coxsackie and adenovirus receptor-negative cells and protection against neutralizing antibodies by HPMA-co-oligolysine copolymer-coated adenovirus. Biomaterials 2011, 32, 9536–9545. [Google Scholar] [CrossRef]
- Kim, P.H.; Kim, T.I.; Yockman, J.W.; Kim, S.W.; Yun, C.O. The effect of surface modification of adenovirus with an arginine-grafted bioreducible polymer on transduction efficiency and immunogenicity in cancer gene therapy. Biomaterials 2010, 31, 1865–1874. [Google Scholar] [CrossRef]
- Kim, P.H.; Kim, J.; Kim, T.I.; Nam, H.Y.; Yockman, J.W.; Kim, M.; Kim, S.W.; Yun, C.O. Bioreducible polymer-conjugated oncolytic adenovirus for hepatoma-specific therapy via systemic administration. Biomaterials 2011, 32, 9328–9342. [Google Scholar] [CrossRef]
- Singh, R.; Tian, B.; Kostarelos, K. Artificial envelopment of nonenveloped viruses: Enhancing adenovirus tumor targeting in vivo. FASEB J. 2008, 22, 3389–3402. [Google Scholar] [CrossRef]
- Singh, R.; Al-Jamal, K.T.; Lacerda, L.; Kostarelos, K. Nanoengineering artificial lipid envelopes around adenovirus by self-assembly. ACS Nano 2008, 2, 1040–1050. [Google Scholar] [CrossRef]
- Yilmazer, A.; Al-Jamal, W.T.; van den Bossche, J.; Kostarelos, K. The effect of artificial lipid envelopment of Adenovirus 5 (Ad5) on liver de-targeting and hepatotoxicity. Biomaterials 2013, 34, 1354–1363. [Google Scholar] [CrossRef]
- Tomko, R.P.; Xu, R.; Philipson, L. HCAR and MCAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 1997, 94, 3352–3356. [Google Scholar] [CrossRef]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins αvβ 3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef]
- Jonsson, M.I.; Lenman, A.E.; Frangsmyr, L.; Nyberg, C.; Abdullahi, M.; Arnberg, N. Coagulation factors IX and X enhance binding and infection of adenovirus types 5 and 31 in human epithelial cells. J. Virol. 2009, 83, 3816–3825. [Google Scholar] [CrossRef]
- Shayakhmetov, D.M.; Gaggar, A.; Ni, S.; Li, Z.Y.; Lieber, A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 2005, 79, 7478–7491. [Google Scholar] [CrossRef]
- Alba, R.; Bradshaw, A.C.; Coughlan, L.; Denby, L.; McDonald, R.A.; Waddington, S.N.; Buckley, S.M.; Greig, J.A.; Parker, A.L.; Miller, A.M.; et al. Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. Blood 2010, 116, 2656–2664. [Google Scholar] [CrossRef]
- Parker, A.L.; Waddington, S.N.; Nicol, C.G.; Shayakhmetov, D.M.; Buckley, S.M.; Denby, L.; Kemball-Cook, G.; Ni, S.; Lieber, A.; McVey, J.H.; et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 2006, 108, 2554–2561. [Google Scholar] [CrossRef]
- Alba, R.; Bradshaw, A.C.; Mestre-Frances, N.; Verdier, J.M.; Henaff, D.; Baker, A.H. Coagulation factor X mediates adenovirus type 5 liver gene transfer in non-human primates (Microcebus murinus). Gene Ther. 2012, 19, 109–113. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Koizumi, N.; Hosono, T.; Ishii-Watabe, A.; Uchida, E.; Utoguchi, N.; Watanabe, Y.; Hayakawa, T. Car- or alphav integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing rgd peptide, do not change the systemic gene transfer properties in mice. Gene Ther. 2002, 9, 769–776. [Google Scholar] [CrossRef]
- Alba, R.; Bradshaw, A.C.; Parker, A.L.; Bhella, D.; Waddington, S.N.; Nicklin, S.A.; van Rooijen, N.; Custers, J.; Goudsmit, J.; Barouch, D.H.; et al. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: Effect of mutagenesis on FX interactions and gene transfer. Blood 2009, 114, 965–971. [Google Scholar] [CrossRef]
- Liu, T.C.; Kirn, D. Gene therapy progress and prospects cancer: Oncolytic viruses. Gene Ther. 2008, 15, 877–884. [Google Scholar] [CrossRef]
- Yamamoto, M.; Curiel, D.T. Current issues and future directions of oncolytic adenoviruses. Mol. Ther. 2010, 18, 243–250. [Google Scholar] [CrossRef]
- Nokisalmi, P.; Pesonen, S.; Escutenaire, S.; Sarkioja, M.; Raki, M.; Cerullo, V.; Laasonen, L.; Alemany, R.; Rojas, J.; Cascallo, M.; et al. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin. Cancer Res. 2010, 16, 3035–3043. [Google Scholar] [CrossRef]
- Cerullo, V.; Vaha-Koskela, M.; Hemminki, A. Oncolytic adenoviruses: A potent form of tumor immunovirotherapy. Oncoimmunology 2012, 1, 979–981. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, J.S.; Kim, S.W.; Yun, C.O. Evolution of oncolytic adenovirus for cancer treatment. Adv. Drug Deliv. Rev. 2012, 64, 720–729. [Google Scholar] [CrossRef]
- DeWeese, T.L.; van der Poel, H.; Li, S.; Mikhak, B.; Drew, R.; Goemann, M.; Hamper, U.; DeJong, R.; Detorie, N.; Rodriguez, R.; et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001, 61, 7464–7472. [Google Scholar]
- Kim, E.; Kim, J.H.; Shin, H.Y.; Lee, H.; Yang, J.M.; Kim, J.; Sohn, J.H.; Kim, H.; Yun, C.O. Ad-mTERT-Delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum. Gene Ther. 2003, 14, 1415–1428. [Google Scholar] [CrossRef]
- Post, D.E.; Van Meir, E.G. A novel hypoxia-inducible factor (HIF) activated oncolytic adenovirus for cancer therapy. Oncogene 2003, 22, 2065–2072. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Wang, Q.; Wang, L.; Yang, H.; Li, M. Increased antitumor capability of fiber-modified adenoviral vector armed with trail against bladder cancers. Mol. Cell. Biochem. 2011, 353, 93–99. [Google Scholar] [CrossRef]
- Granio, O.; Ashbourne Excoffon, K.J.; Henning, P.; Melin, P.; Norez, C.; Gonzalez, G.; Karp, P.H.; Magnusson, M.K.; Habib, N.; Lindholm, L.; et al. Adenovirus 5-fiber 35 chimeric vector mediates efficient apical correction of the cystic fibrosis transmembrane conductance regulator defect in cystic fibrosis primary airway epithelia. Hum. Gene Ther. 2010, 21, 251–269. [Google Scholar] [CrossRef]
- Diaconu, I.; Denby, L.; Pesonen, S.; Cerullo, V.; Bauerschmitz, G.J.; Guse, K.; Rajecki, M.; Dias, J.D.; Taari, K.; Kanerva, A.; et al. Serotype chimeric and fiber-mutated adenovirus Ad5/19p-HIT for targeting renal cancer and untargeting the liver. Hum. Gene Ther. 2009, 20, 611–620. [Google Scholar] [CrossRef]
- Rodriguez, E.; Romero, C.; Rio, A.; Miralles, M.; Raventos, A.; Planells, L.; Burgueno, J.F.; Hamada, H.; Perales, J.C.; Bosch, A.; et al. Short-fiber protein of Ad40 confers enteric tropism and protection against acidic gastrointestinal conditions. Hum. Gene Ther. Methods 2013, 24, 195–204. [Google Scholar] [CrossRef]
- Smith, T.A.; Idamakanti, N.; Rollence, M.L.; Marshall-Neff, J.; Kim, J.; Mulgrew, K.; Nemerow, G.R.; Kaleko, M.; Stevenson, S.C. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 2003, 14, 777–787. [Google Scholar] [CrossRef]
- Kritz, A.B.; Nicol, C.G.; Dishart, K.L.; Nelson, R.; Holbeck, S.; von Seggern, D.J.; Work, L.M.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Adenovirus 5 fibers mutated at the putative HSPG-bindingsite show restricted retargeting with targeting peptides in the HI loop. Mol. Ther. 2007, 15, 741–749. [Google Scholar]
- Bayo-Puxan, N.; Cascallo, M.; Gros, A.; Huch, M.; Fillat, C.; Alemany, R. Role of the putative heparan sulfate glycosaminoglycan-binding site of the adenovirus type 5 fiber shaft on liver detargeting and knob-mediated retargeting. J. Gen. Virol. 2006, 87, 2487–2495. [Google Scholar] [CrossRef]
- Wu, E.; Pache, L.; von Seggern, D.J.; Mullen, T.M.; Mikyas, Y.; Stewart, P.L.; Nemerow, G.R. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J. Virol. 2003, 77, 7225–7235. [Google Scholar] [CrossRef]
- Koizumi, N.; Kawabata, K.; Sakurai, F.; Watanabe, Y.; Hayakawa, T.; Mizuguchi, H. Modified adenoviral vectors ablated for coxsackievirus-adenovirus receptor, alphav integrin, and heparan sulfate binding reduce in vivo tissue transduction and toxicity. Hum. Gene Ther. 2006, 17, 264–279. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, K.; Hamada, H. Reduction of natural adenovirus tropism to the liver by both ablation of fiber-coxsackievirus and adenovirus receptor interaction and use of replaceable short fiber. J. Virol. 2003, 77, 2512–2521. [Google Scholar] [CrossRef]
- Law, L.K.; Davidson, B.L. What does it take to bind car? Mol. Ther. 2005, 12, 599–609. [Google Scholar] [CrossRef]
- Dechecchi, M.C.; Tamanini, A.; Bonizzato, A.; Cabrini, G. Heparan sulfate glycosaminoglycans are involved in Adenovirus type 5 and 2-host cell interactions. Virology 2000, 268, 382–390. [Google Scholar] [CrossRef]
- Hall, K.; Zajdel, M.E.B.; Blair, G.E. Defining the role of CD46, CD80 and CD86 in mediating Adenovirus type 3 fiber interactions with host cells. Virology 2009, 392, 222–229. [Google Scholar] [CrossRef]
- Hara, T.; Kojima, A.; Fukuda, H.; Masaoka, T.; Fukumori, Y.; Matsumoto, M.; Seya, T. Levels of complement regulatory proteins, CD35 (CR1), CD46 (MCP) and CD55 (DAF) in human haematological malignancies. Br. J. Haematol. 1992, 82, 368–373. [Google Scholar] [CrossRef]
- Thorsteinsson, L.; O'Dowd, G.M.; Harrington, P.M.; Johnson, P.M. The complement regulatory proteins CD46 and CD59, but not CD55, are highly expressed by glandular epithelium of human breast and colorectal tumour tissues. Acta Pathol. Microbiol. Immunol. Scand. Suppl. 1998, 106, 869–878. [Google Scholar] [CrossRef]
- Murakami, M.; Ugai, H.; Belousova, N.; Pereboev, A.; Dent, P.; Fisher, P.B.; Everts, M.; Curiel, D.T. Chimeric Adenoviral vectors incorporating a fiber of human adenovirus 3 efficiently mediate gene transfer into prostate cancer cells. Prostate 2010, 70, 362–376. [Google Scholar]
- Kim, K.H.; Ryan, M.J.; Estep, J.E.; Miniard, B.M.; Rudge, T.L.; Peggins, J.O.; Broadt, T.L.; Wang, M.; Preuss, M.A.; Siegal, G.P.; et al. A new generation of serotype chimeric infectivity-enhanced conditionally replicative adenovirals: The safety profile of Ad5/3-Δ24 in advance of a phase I clinical trial in ovarian cancer patients. Hum. Gene Ther. 2011, 22, 821–828. [Google Scholar] [CrossRef]
- Kim, K.H.; Dmitriev, I.P.; Saddekni, S.; Kashentseva, E.A.; Harris, R.D.; Aurigemma, R.; Bae, S.; Singh, K.P.; Siegal, G.P.; Curiel, D.T.; et al. A phase I clinical trial of Ad5/3-Δ24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol. Oncol. 2013, 130, 518–524. [Google Scholar] [CrossRef]
- Van de Ven, R.; Lindenberg, J.J.; Oosterhoff, D.; van den Tol, M.P.; Rosalia, R.A.; Murakami, M.; Everts, M.; Scheffer, G.L.; Scheper, R.J.; de Gruijl, T.D.; et al. Selective transduction of mature DC in human skin and lymph nodes by CD80/CD86-targeted fiber-modified adenovirus-5/3. J. Immunother. 2009, 32, 895–906. [Google Scholar] [CrossRef]
- Koski, A.; Karli, E.; Kipar, A.; Escutenaire, S.; Kanerva, A.; Hemminki, A. Mutation of the fiber shaft heparan sulphate binding site of a 5/3 chimeric adenovirus reduces liver tropism. PLoS One 2013, 8, e60032. [Google Scholar]
- Hogg, R.T.; Thorpe, P.; Gerard, R.D. Retargeting adenoviral vectors to improve gene transfer into tumors. Cancer Gene Ther. 2011, 18, 275–287. [Google Scholar] [CrossRef]
- Belousova, N.; Mikheeva, G.; Gelovani, J.; Krasnykh, V. Modification of Adenovirus capsid with a designed protein ligand yields a gene vector targeted to a major molecular marker of cancer. J. Virol. 2008, 82, 630–637. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Ruoslahti, E. Tumor-penetrating peptides. Front. Oncol. 2013, 3. [Google Scholar] [CrossRef]
- Coughlan, L.; Vallath, S.; Saha, A.; Flak, M.; McNeish, I.A.; Vassaux, G.; Marshall, J.F.; Hart, I.R.; Thomas, G.J. In vivo retargeting of adenovirus type 5 to αvβ6 integrin results in reduced hepatotoxicity and improved tumor uptake following systemic delivery. J. Virol. 2009, 83, 6416–6428. [Google Scholar] [CrossRef]
- Pesonen, S.; Diaconu, I.; Cerullo, V.; Escutenaire, S.; Raki, M.; Kangasniemi, L.; Nokisalmi, P.; Dotti, G.; Guse, K.; Laasonen, L.; et al. Integrin targeted oncolytic adenoviruses Ad5-d24-RGD and AD5-RGD-d24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int. J. Cancer 2012, 130, 1937–1947. [Google Scholar] [CrossRef]
- Rojas, J.J.; Gimenez-Alejandre, M.; Gil-Hoyos, R.; Cascallo, M.; Alemany, R. Improved systemic antitumor therapy with oncolytic adenoviruses by replacing the fiber shaft HSG-binding domain with RGD. Gene Ther. 2012, 19, 453–457. [Google Scholar] [CrossRef]
- Matsui, H.; Sakurai, F.; Katayama, K.; Kurachi, S.; Tashiro, K.; Sugio, K.; Kawabata, K.; Mizuguchi, H. Enhanced transduction efficiency of fiber-substituted adenovirus vectors by the incorporation of rgd peptides in two distinct regions of the adenovirus serotype 35 fiber knob. Virus Res. 2011, 155, 48–54. [Google Scholar] [CrossRef]
- Gamble, L.J.; Ugai, H.; Wang, M.; Borovjagin, A.V.; Matthews, Q.L. Therapeutic efficacy of an oncolytic adenovirus containing rgd ligand in minor capsid protein IX and fiber, Δ24doublergd, in an ovarian cancer model. J. Mol. Biochem. 2012, 1, 26–39. [Google Scholar]
- Wu, H.; Han, T.; Belousova, N.; Krasnykh, V.; Kashentseva, E.; Dmitriev, I.; Kataram, M.; Mahasreshti, P.J.; Curiel, D.T. Identification of sites in adenovirus hexon for foreign peptide incorporation. J. Virol. 2005, 79, 3382–3390. [Google Scholar] [CrossRef]
- Di, B.; Mao, Q.; Zhao, J.; Li, X.; Wang, D.; Xia, H. A rapid generation of adenovirus vector with a genetic modification in hexon protein. J. Biotechnol. 2012, 157, 373–378. [Google Scholar] [CrossRef]
- Doronin, K.; Shashkova, E.V.; May, S.M.; Hofherr, S.E.; Barry, M.A. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum. Gene Ther. 2009, 20, 975–988. [Google Scholar] [CrossRef]
- Mok, H.; Palmer, D.J.; Ng, P.; Barry, M.A. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 2005, 11, 66–79. [Google Scholar]
- Hofherr, S.E.; Shashkova, E.V.; Weaver, E.A.; Khare, R.; Barry, M.A. Modification of adenoviralvectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol. Ther. 2008, 16, 1276–1282. [Google Scholar] [CrossRef]
- Croyle, M.A.; Chirmule, N.; Zhang, Y.; Wilson, J.M. Pegylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum. Gene Ther. 2002, 13, 1887–1900. [Google Scholar] [CrossRef]
- Hofherr, S.E.; Mok, H.; Gushiken, F.C.; Lopez, J.A.; Barry, M.A. Polyethylene glycol modification of adenovirus reduces platelet activation, endothelial cell activation, and thrombocytopenia. Hum. Gene Ther. 2007, 18, 837–848. [Google Scholar] [CrossRef]
- Morral, N.; O’Neal, W.K.; Rice, K.; Leland, M.M.; Piedra, P.A.; Aguilar-Cordova, E.; Carey, K.D.; Beaudet, A.L.; Langston, C. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an Adenoviral vector in baboons. Hum. Gene Ther. 2002, 13, 143–154. [Google Scholar] [CrossRef]
- Matsui, H.; Sakurai, F.; Katayama, K.; Yamaguchi, T.; Okamoto, S.; Takahira, K.; Tachibana, M.; Nakagawa, S.; Mizuguchi, H. A hexon-specific pegylated adenovirus vector utilizing blood coagulation factor X. Biomaterials 2012, 33, 3743–3755. [Google Scholar] [CrossRef]
- Suzuki-Kouyama, E.; Katayama, K.; Sakurai, F.; Yamaguchi, T.; Kurachi, S.; Kawabata, K.; Nakagawa, S.; Mizuguchi, H. Hexon-specific pegylated adenovirus vectors utilizing avidin-biotin interaction. Biomaterials 2011, 32, 1724–1730. [Google Scholar] [CrossRef]
- Green, N.K.; Herbert, C.W.; Hale, S.J.; Hale, A.B.; Mautner, V.; Harkins, R.; Hermiston, T.; Ulbrich, K.; Fisher, K.D.; Seymour, L.W. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004, 11, 1256–1263. [Google Scholar] [CrossRef]
- Wang, I.J.; Jhuang, M.C.; Chen, Y.H.; Yeh, L.K.; Liu, C.Y.; Young, T.H. Chitosan modification of adenovirus to modify transfection efficiency in bovine corneal epithelial cells. PLoS One 2010, 5, e12085. [Google Scholar]
- Grunwald, G.K.; Vetter, A.; Klutz, K.; Willhauck, M.J.; Schwenk, N.; Senekowitsch-Schmidtke, R.; Schwaiger, M.; Zach, C.; Wagner, E.; Goke, B.; et al. Systemic image-guided liver cancer radiovirotherapy using dendrimer-coated adenovirus encoding the sodium iodide symporter as theranostic gene. J. Nuclear Med. 2013, 54, 1450–1457. [Google Scholar] [CrossRef]
- Maksimenko, A.V.; Mandrouguine, V.; Gottikh, M.B.; Bertrand, J.R.; Majoral, J.P.; Malvy, C. Optimisation of dendrimer-mediated gene transfer by anionic oligomers. J. Gene Med. 2003, 5, 61–71. [Google Scholar]
- Vetter, A.; Virdi, K.S.; Espenlaub, S.; Rodl, W.; Wagner, E.; Holm, P.S.; Scheu, C.; Kreppel, F.; Spitzweg, C.; Ogris, M. Adenoviral vectors coated with pamam dendrimer conjugates allow car independent virus uptake and targeting to the egf receptor. Mol. Pharm. 2013, 10, 606–618. [Google Scholar] [CrossRef]
- Pandori, M.W.; Hobson, D.A.; Sano, T. Adenovirus-microbead conjugates possess enhanced infectivity: A new strategy for localized gene delivery. Virology 2002, 299, 204–212. [Google Scholar] [CrossRef]
- Sapet, C.; Pellegrino, C.; Laurent, N.; Sicard, F.; Zelphati, O. Magnetic nanoparticles enhance adenovirus transduction in vitro and in vivo. Pharm. Res. 2012, 29, 1203–1218. [Google Scholar] [CrossRef]
- Hoffman, J.A.; Giraudo, E.; Singh, M.; Zhang, L.; Inoue, M.; Porkka, K.; Hanahan, D.; Ruoslahti, E. Progressive vascular changes in a transgenic mouse model of squamous cell carcinoma. Cancer Cell 2003, 4, 383–391. [Google Scholar] [CrossRef]
- Yao, X.L.; Yoshioka, Y.; Ruan, G.X.; Chen, Y.Z.; Mizuguchi, H.; Mukai, Y.; Okada, N.; Gao, J.Q.; Nakagawa, S. Optimization and internalization mechanisms of pegylated adenovirus vector with targeting peptide for cancer gene therapy. Biomacromolecules 2012, 13, 2402–2409. [Google Scholar] [CrossRef]
- Yao, X.; Yoshioka, Y.; Morishige, T.; Eto, Y.; Narimatsu, S.; Kawai, Y.; Mizuguchi, H.; Gao, J.Q.; Mukai, Y.; Okada, N.; et al. Tumor vascular targeted delivery of polymer-conjugated adenovirus vector for cancer gene therapy. Mol. Ther. 2011, 19, 1619–1625. [Google Scholar] [CrossRef]
- Xiong, Z.; Cheng, Z.; Zhang, X.; Patel, M.; Wu, J.C.; Gambhir, S.S.; Chen, X. Imaging chemically modified adenovirus for targeting tumors expressing integrin αvβ3 in living mice with mutant herpes simplex virus type 1 thymidine kinase pet reporter gene. J. Nuclear Med. 2006, 47, 130–139. [Google Scholar]
- King, W.J.; Krebsbach, P.H. Cyclic-RGD peptides increase the adenoviral transduction of human mesenchymal stem cells. Stem Cells Dev. 2013, 22, 679–686. [Google Scholar] [CrossRef]
- Black, P.C.; Agarwal, P.K.; Dinney, C.P. Targeted therapies in bladder cancer—An update. Urol. Oncol. 2007, 25, 433–438. [Google Scholar] [CrossRef]
- Bonsted, A.; Engesaeter, B.O.; Hogset, A.; Maelandsmo, G.M.; Prasmickaite, L.; D’Oliveira, C.; Hennink, W.E.; van Steenis, J.H.; Berg, K. Photochemically enhanced transduction of polymer-complexed adenovirus targeted to the epidermal growth factor receptor. J. Gene Med. 2006, 8, 286–297. [Google Scholar] [CrossRef]
- Hogset, A.; Engesaeter, B.O.; Prasmickaite, L.; Berg, K.; Fodstad, O.; Maelandsmo, G.M. Light-induced adenovirus gene transfer, an efficient and specific gene delivery technology for cancer gene therapy. Cancer Gene Ther. 2002, 9, 365–371. [Google Scholar] [CrossRef]
- Tang, D.C.; Jennelle, R.S.; Shi, Z.; Garver, R.I., Jr.; Carbone, D.P.; Loya, F.; Chang, C.H.; Curiel, D.T. Overexpression of adenovirus-encoded transgenes from the cytomegalovirus immediate early promoter in irradiated tumor cells. Human Gene Ther. 1997, 8, 2117–2124. [Google Scholar] [CrossRef]
- Engesaeter, B.O.; Bonsted, A.; Berg, K.; Hogset, A.; Engebraten, O.; Fodstad, O.; Curiel, D.T.; Maelandsmo, G.M. PCI-enhanced adenoviral transduction employs the known uptake mechanism of adenoviral particles. Cancer Gene Ther. 2005, 12, 439–448. [Google Scholar]
- Morrison, J.; Briggs, S.S.; Green, N.; Fisher, K.; Subr, V.; Ulbrich, K.; Kehoe, S.; Seymour, L.W. Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol. Ther. 2008, 16, 244–251. [Google Scholar] [CrossRef]
- Walters, C.L.; Arend, R.C.; Armstrong, D.K.; Naumann, R.W.; Alvarez, R.D. Folate and folate receptor α antagonists mechanism of action in ovarian cancer. Gynecol. Oncol. 2013, 131, 493–498. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, H.; Xu, Y.; Liu, T.; Chen, S.; Wang, J.; Zhang, T. Expression of folate receptors in nasopharyngeal and laryngeal carcinoma and folate receptor-mediated endocytosis by molecular targeted nanomedicine. Int. J. Nanomed. 2013, 8, 2443–2451. [Google Scholar]
- Kwon, O.J.; Kang, E.; Choi, J.W.; Kim, S.W.; Yun, C.O. Therapeutic targeting of chitosan-peg-folate-complexed oncolytic adenovirus for active and systemic cancer gene therapy. J. Control. Release 2013, 169, 257–265. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhu, N.L.; Chua, J.; Swenson, S.; Costa, F.K.; Schmitmeier, S.; Sosnowski, B.A.; Shichinohe, T.; Kasahara, N.; Chen, T.C. Retargeting of adenoviral vector using basic fibroblast growth factor ligand for malignant glioma gene therapy. J. Neurosurg. 2005, 103, 1058–1066. [Google Scholar] [CrossRef]
- Lanciotti, J.; Antonius, S.; Doukas, J.; Sosnowski, B.; Pierce, G.; Gregory, R.; Wadsworth, S.; O’Riordan, C. Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Mol. Ther. 2003, 8, 99–107. [Google Scholar] [CrossRef]
- Green, N.K.; Morrison, J.; Hale, S.; Briggs, S.S.; Stevenson, M.; Subr, V.; Ulbrich, K.; Chandler, L.; Mautner, V.; Seymour, L.W.; et al. Retargeting polymer-coated adenovirus to the FGF receptor allows productive infection and mediates efficacy in a peritoneal model of human ovarian cancer. J. Gene Med. 2008, 10, 280–289. [Google Scholar] [CrossRef]
- Pearson, S.; Jia, H.; Kandachi, K. China approves first gene therapy. Nat. Biotechnol. 2004, 22, 3–4. [Google Scholar] [CrossRef]
- Raty, J.K.; Pikkarainen, J.T.; Wirth, T.; Yla-Herttuala, S. Gene therapy: The first approved gene-based medicines, molecular mechanisms and clinical indications. Curr. Mol. Pharmacol. 2008, 1, 13–23. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Capasso, C.; Garofalo, M.; Hirvinen, M.; Cerullo, V. The Evolution of Adenoviral Vectors through Genetic and Chemical Surface Modifications. Viruses 2014, 6, 832-855. https://doi.org/10.3390/v6020832
Capasso C, Garofalo M, Hirvinen M, Cerullo V. The Evolution of Adenoviral Vectors through Genetic and Chemical Surface Modifications. Viruses. 2014; 6(2):832-855. https://doi.org/10.3390/v6020832
Chicago/Turabian StyleCapasso, Cristian, Mariangela Garofalo, Mari Hirvinen, and Vincenzo Cerullo. 2014. "The Evolution of Adenoviral Vectors through Genetic and Chemical Surface Modifications" Viruses 6, no. 2: 832-855. https://doi.org/10.3390/v6020832



