Modeling of Anopheles minimus Mosquito NADPH-Cytochrome P450 Oxidoreductase (CYPOR) and Mutagenesis Analysis
Abstract
:1. Introduction
2. Results and Discussion
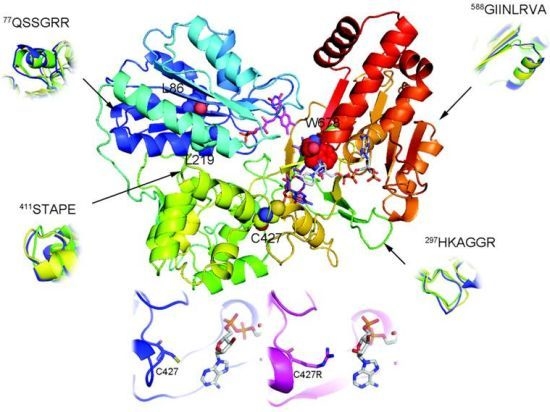
2.1. Overall Structure of AnCYPOR
2.2. Predicted FAD-Binding Region
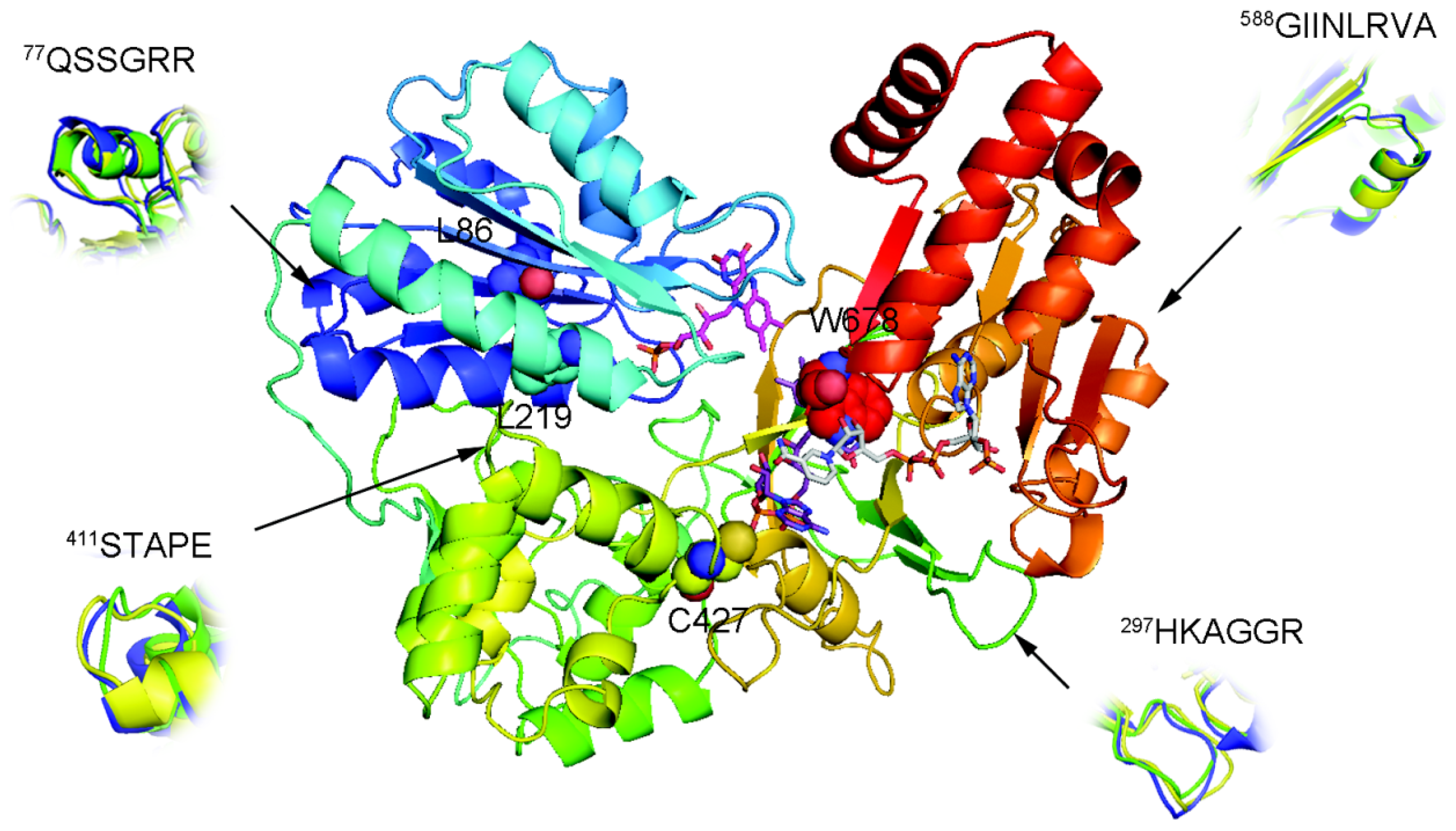
2.3. FAD/NADPH Binding Residues in AnCYPOR
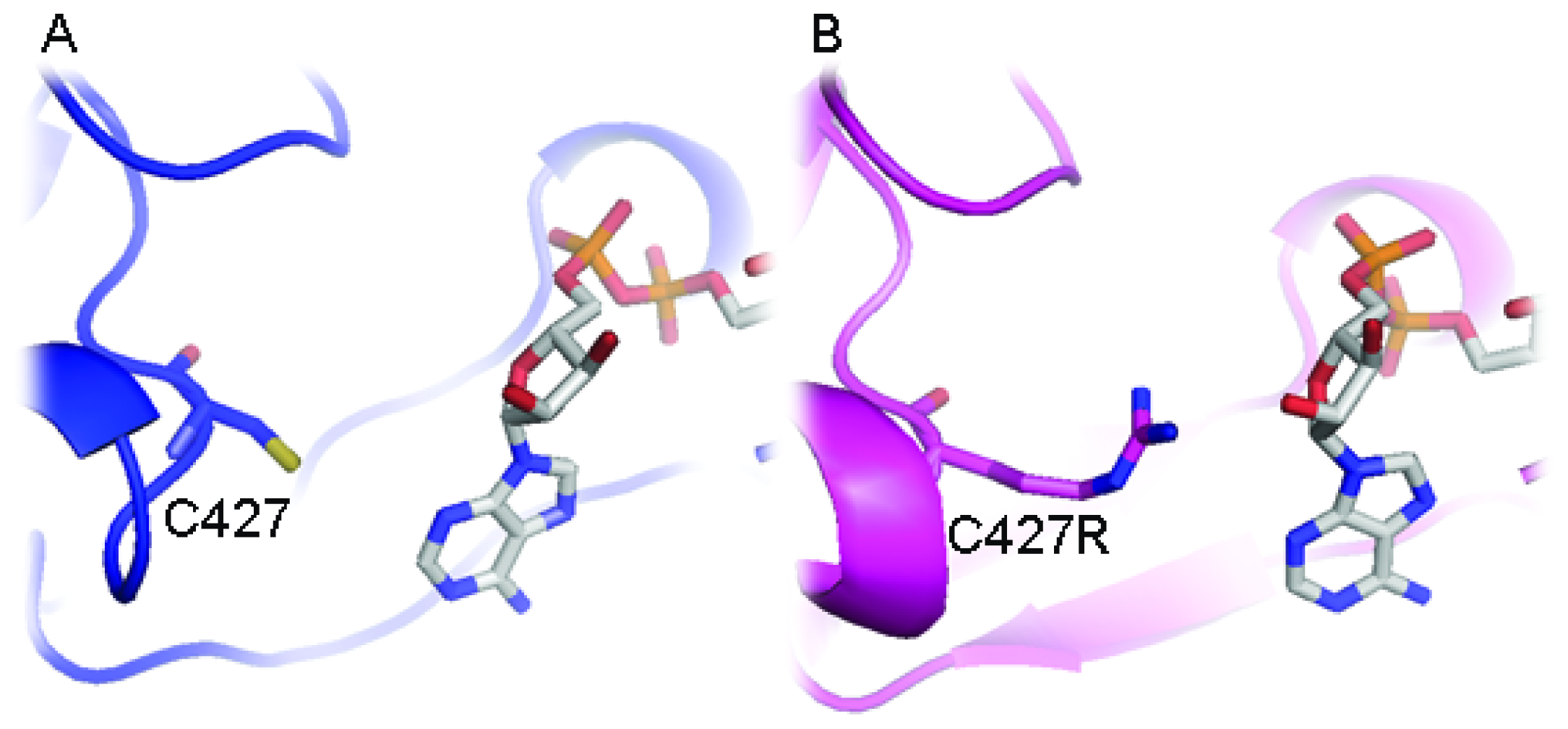
2.4. Electron Transfer Step Is a Rate-Limiting Step in Mosquito P450 Metabolism
3. Experimental Section
3.1. Materials
3.2. Structure Prediction of Wild-Type and Mutant CYPOR
3.3. Site-Directed Mutagenesis of AnCYPORs
3.4. Expression and Purification of AnCYPOR and Rat CYPOR Enzymes
3.5. Expression and Purification of CYP6AA3 from Insect-Baculovirus System
3.6. Measurement of Flavin Contents and Activity Assay
3.7. CYP6AA3-Mediated BROD Assay
4. Conclusions
Supplementary Information
ijms-14-01788-s001.pdfAcknowledgments
References
- Scott, J.F. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol 1999, 29, 757–777. [Google Scholar]
- Rivero, A.; Vézilier, J.; Weill, M.; Read, A.F.; Gandon, S. Insecticide control of vector-borne diseases: When is insecticide resistance a problem? PLoS Pathog 2010, 6, e1001000. [Google Scholar]
- Chareonviriyaphap, T.; Aum-Aung, B.; Ratanatham, S. Current insecticide resistance pattern in mosquito vectors in Thailand. Southeast Asian J. Trop. Med. Public Health 1999, 30, 184–194. [Google Scholar]
- Feyereisen, R. Insect P450 enzyme. Annu. Rev. Entomol 1999, 44, 507–533. [Google Scholar]
- Rongnoparut, P.; Boonsuepsakul, S.; Chareonviriyaphap, T.; Thanomsing, N. Molecular cloning and expression of cytochrome P450, CYP6P5 and CYP6AA2, from Anopheles minimus strain resistant to deltamethrin. J. Vector Ecol 2003, 2, 150–158. [Google Scholar]
- Rodpradit, P.; Boonsuepsakul, S.; Chareonviriyaphap, T.; Bangs, M.J.; Rongnoparut, P. Cytochrome P450 genes: Molecular cloning and over expression in a pyrethroid-resistant strain of Anopheles minimus mosquito. J. Am. Mosq. Control Assoc 2005, 21, 71–79. [Google Scholar]
- Kaewpa, D.; Boonsuepsakul, S.; Rongnoparut, P. Functional expression of mosquito NADPH-cytochrome P450 reductase in Escherichia coli. J. Econ. Entomol 2007, 100, 946–953. [Google Scholar]
- Boonsuepsakul, S.; Luepromchai, E.; Rongnoparut, P. Characterization of Anopheles minimus CYP6AA3 expressed in a recombinant baculovirus system. Arch. Insect Biochem. Physiol 2008, 69, 13–21. [Google Scholar]
- Duangkaew, P.; Pet-Huan, S.; Kaewpa, D.; Boonsuepsakul, S.; Sarapusit, S.; Rongnoparut, P. Characterization of mosquito CYP6P7 and CYP6AA3: Differences in substrate preference and kinetic properties. Arch. Insect Biochem. Physiol 2011, 76, 1–13. [Google Scholar]
- Wang, M.; Roberts, D.L.; Paschke, R.; Shea, T.M.; Masters, B.S.S.; Kim, J.J.P. Three-Dimensional structure of NADPH-cytochrome P450 reductase: Prototype for FMN- and FAD-containing enzymes. Proc. Natl. Acad. Sci. USA 1997, 94, 8411–8416. [Google Scholar]
- Murataliev, M.B.; Feyereisen, R.; Walker, F.A. Electron transfer by diflavin reductase. Biochim. Biophys. Acta 2004, 1698, 1–26. [Google Scholar]
- Paine, M.J.I.; Scrutton, N.S.; Munro, A.W.; Gutierrez, A.; Roberts, G.C.K.; Wolf, C.R. Electron transfer partner of cytochrome P450. In Cytochrome P450: Structure, Mechanism and Biochemistry, 3rd ed; Ortiz de Montellano, P.R., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005; pp. 115–148. [Google Scholar]
- Lycett, G.L.; McLaughlin, L.A.; Ranson, H.; Hemingway, J.; Kafatos, F.C.; Loukeris, T.G.; Paine, M.J.I. Anopheles gambiae P450 reductase is highly expressed in oeocytes and in vivo knockdown increases permethrin susceptibility. Insect Mol. Biol 2006, 15, 321–327. [Google Scholar]
- Sarapusit, S.; Xia, C.W.; Misra, I.; Rongnoparut, P.; Kim, J.J.P. NADPH-cytochrome P450-oxidoreductase from the mosquito Anopheles minimus: Kinetic studies and the influence of leu86 and leu219 on cofactor binding and protein stability. Arch. Biochem. Biophys 2008, 477, 53–59. [Google Scholar]
- Sarapusit, S.; Pet-huan, S.; Rongnoparut, P. Mosquito NADPH-cytochrome P450-oxidoreductase mutation: Kinetic and role in CYP6AA3-mediated deltamethrin metabolism. Arch. Insect Biochem. Physiol 2010, 73, 232–244. [Google Scholar]
- Mayer, R.T.; Durrant, J.L. Preparation of homogenous NADPH-cytochrome (P450) reductase from house flies using affinity chromatography techniques. J. Biol. Chem 1979, 254, 756–761. [Google Scholar]
- Shen, A.L.; Porter, T.D.; Wilson, T.E.; Kasper, C.B. Structural analysis of the FMN binding domain of NADPH-cytochrome P450 oxidoreductase by site-directed mutagenesis. J. Biol. Chem 1989, 254, 7584–7589. [Google Scholar]
- Lian, L.-Y.; Widdowson, P.; McLaughlin, L.A.; Paine, M.J.I. Biochemical comparison of Anopheles gambiae and human NADPH P450 reductases reveals different 2′-5′-ADP and FMN binding traits. PLoS One 2011, 6, e20574. [Google Scholar]
- Hubbard, P.A.; Shen, A.L.; Paschke, R.; Kasper, C.B.; Kim, J.J.P. NADPH-cytochrome P450 oxidoreductase: Structural basis for hydride and electron transfer. J. Biol. Chem 2001, 276, 29163–29170. [Google Scholar]
- Hamdane, D.; Xia, C.; Im, S.C.; Zhang, H.; Kim, J.J.P.; Waskell, L. Structure and function of an NADPH-cytochrome P450 oxidoreductase in an open conformation capable of reducing cytochrome P450. J. Biol. Chem 2009, 284, 11374–11384. [Google Scholar]
- Xia, C.; Panda, S.P.; Marohnic, C.C.; Martasek, P.; Masters, B.S.; Kim, J.J.P. Structural basis for human NADPH-cytochrome P450 oxidoreductase deficiency. Proc. Natl. Acad. Sci. USA 2011, 108, 13486–13489. [Google Scholar]
- Marohnic, C.; Panda, S.; Martisek, P.; Masters, B.S.S. Diminished FAD binding in the Y459H and V492E Antley-Bixler syndrome mutants of human cytochrome P450 reductase. J. Biol. Chem 2006, 281, 35975–35982. [Google Scholar]
- Kranendonk, M.; Marochic, C.; Panda, S.P.; Duarte, M.P.; Oliveira, J.S.; Masters, B.S.S. Impairment of CYP1A2-mediated xenobiotic metabolism by Antley-Bixler syndrome variants of cytochrome P450 oxidoreductase. Arch. Biochem. Biophys 2008, 475, 93–99. [Google Scholar]
- Dőhr, O.; Paine, M.J.; Friedberg, T.; Robert, G.C.; Wolf, R. Engineering of a functional human NADH-dependent cytochrome P450 system. Proc. Natl. Acad. Sci. USA 2001, 98, 81–86. [Google Scholar]
- Elmore, C.L.; Porter, T.D. Modification of the nucleotide cofactor binding site of cytochrome P450 reductase to enhance turnover with NADH in vivo. J. Biol. Chem 2002, 277, 48960–48964. [Google Scholar]
- Sarapusit, S. The study on the NADPH-Cytochrome P450 oxidoreductase from Anopheles minimus mosquito. Ph.D. Thesis, Mahidol Univerisity, Thailand, 2009. [Google Scholar]
- Gomes, A.M.; Winter, S.; Klein, K.; Turpeinen, M.; Schaeffeler, E.; Schwab, M.; Zanger, U.M. Pharmacogenomics of human liver cytochrome P450 oxidoreductase: Multifactorial analysis and impact on microsomal drug oxidation. Pharmacogenomics J 2009, 10, 579–599. [Google Scholar]
- Flűck, C.E.; Tajima, T.; Pandey, A.V.; Arlt, W.; Okuhara, K.; Verge, C.F.; Jabs, E.W.; Mendonca, B.B.; Fujieda, K.; Miller, W.L. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat. Genet 2004, 36, 228–230. [Google Scholar]
- Pandey, A.V.; Kempna, P.; Hofer, G.; Mullis, P.E.; Flűck, C.E. Modulation of human CYP19A1 activity by mutant NADPH P450 oxidoreducatse. Mol. Endocrinol 2007, 21, 2579–2595. [Google Scholar]
- Palma, B.B.; Sousa, E.M.S.; Vosmeer, C.R.; Lastdrager, J.; Rueff, J.; Vermeulen, N.P.; Kranendonk, M. Functional characterization of eight human cytochrome P450 1A2 gene variants by recombinant protein expression. Pharmacogenomics J 2010, 10, 478–488. [Google Scholar]
- Pandey, A.V.; Flűck, C.E.; Mullis, P.E. Altered heme catabolism by heme oxygenase-1 caused by mutations in human NADPH cytochrome P450 reductase. Biochem. Biophys. Res. Commun 2010, 400, 374–378. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res 2000, 28, 235–242. [Google Scholar]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol 1993, 234, 779–815. [Google Scholar]
- Shen, M.Y.; Sali, A. Statistical potential for assessment and prediction of protein structures. Protein Sci 2006, 15, 2507–2524. [Google Scholar]
- Samudrala, R.; Moult, J. An all-atom distance-dependent conditional probability discriminatory function for protein structure prediction. J. Mol. Biol 1998, 275, 895–916. [Google Scholar]
- Case, D.A.; Darden, T.A.; Cheatham, T.E.; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Crowley, M.; Walker, R.C.; Zhang, W.; et al. AMBER 10; University of California: San Francisco, CA, USA, 2008. [Google Scholar]
- Sippl, M.J. Recognition of errors in three-dimensional structures of proteins. Proteins 1993, 17, 355–362. [Google Scholar]
- Wiederstein, M.; Sippl, M.J. ProSA-Web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 2007, 35, W407–W410. [Google Scholar]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Cryst 1993, 26, 283–291. [Google Scholar]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsome. I. Evidence for its hemoprotein nature. J. Biol. Chem 1964, 239, 48–54. [Google Scholar]
- Aliverti, A.; Curti, B.; Varoni, M.A. Identifying and quantitating FAD and FMN in simple and in iron-sulfur-containing flavoproteins. In Flavoprotein Protocols, 2nd ed; Chapman, S.K., Reid, G.A., Eds.; Humana Press: Totowa, NJ, USA, 1999; pp. 9–23. [Google Scholar]
- Voznesensky, A.I.; Schenkman, J.B.; Pernecky, S.J.; Coon, M.J. The NH2-teminal region of rabbit CYP2E1 is not essential for interaction with NADPH-cytochrome P450 reductase. Biochem. Biophys. Res. Commun 1994, 203, 156–161. [Google Scholar]
- Lamb, D.C.; Warrilow, A.G.S.; Venkateswarlu, K.; Kelly, D.E.; Kelly, S.L. Activity and kinetic mechanism of native and soluble NADPH-cytochrome P450 reductase. Biochem. Biophys. Res. Commun 2001, 286, 48–54. [Google Scholar]
| Enzyme | FMN a | FAD a |
|---|---|---|
| Wild-type-Δ55AnCYPOR | 0.52 ± 0.01 | 0.64 ± 0.03 |
| L86F/L219F-Δ55AnCYPOR | 0.97 ± 0.02 | 0.56 ± 0.01 |
| L86F/L219F/C427R-Δ55AnCYPOR | 0.98 ± 0.01 | 0.82 ± 0.02 |
| L86F/L219F/W678A-Δ55AnCYPOR | 0.96 ± 0.04 | 0.55 ± 0.02 |
| L86F/L219F/W678H-Δ55AnCYPOR | 0.97 ± 0.02 | 0.57 ± 0.04 |
| Δ63AgCYPOR b | 0.72 ± 0.01 | 0.80 ± 0.01 |
| Human CYPOR b | 0.88 ± 0.01 | 0.92 ± 0.02 |
| Enzyme | Kinetic constant (μM) a | ||
|---|---|---|---|
| Cytochrome c Km | NADPH Km | NADH Km | |
| Δ55AnCYPOR | |||
| -wt | 27.39 ± 1.41 | 9.61 ± 0.30 | 8.40 ± 0.10 |
| -L86F/L219F | 16.41 ± 2.22 | 6.49 ± 1.19 | 11.64 ± 1.44 |
| -L86F/L219F/C427R | 17.92 ± 1.79 | 7.02 ± 1.75 | 6.67 ± 2.23 |
| -L86F/L219F/W678A | 16.24 ± 1.56 | 3.34 ± 0.83 | 7.08 ± 2.06 |
| -L86F/L219F/W678H | 18.43 ± 2.12 | 1.91 ± 0.44 | 7.72 ± 1.33 |
| CYPOR constructs | Cytochrome c reduction activity (U/mg protein) a | CYP3AA3-mediated BROD activity (pmole resorufin produced/min/pmol P450) a | |
|---|---|---|---|
| Km (μM) | Vmax (min−1) | ||
| wt-AnCYPOR | 0.35 ± 0.02 | 1.90 ± 0.61 | 3.10 ± 0.38 |
| L86F/L219F-AnCYPOR | 0.87 ± 0.04 | 1.89 ± 0.53 | 7.36 ± 0.78 |
| L86F/L219F/C427R-AnCYPOR | 1.62 ± 0.09 | 1.73 ± 0.17 | 10.20 ± 0.37 |
| Rat CYPOR | 18.55 ± 0.42 | 1.69 ± 0.24 | 17.40 ± 0.90 |
| Constructs | Primer | Sequence |
|---|---|---|
| C427R | sense | 5′-GGTACAAGACAGCCGCCGGAACGTAGTGCA-3′ |
| anti-sense | 5′-TGCACTACGTTCCGGCGGCTGTCTTGTACC-3′ | |
| W678H | sense | 5′-ACGTTACTCGGCGGACGTGCACAGCTAATCGACGGGCACA-3′ |
| anti-sense | 5′-TGTGCCCGTCGATTAGCTGTGCACGTCCGCCGAGTAACGT-3′ | |
| W678A | sense | 5′-ACGTTACTCGGCGGACGTGGCAAGCTAATCGACGGGCACA-3′ |
| anti-sense | 5′-TGTGCCCGTCGATTAGCTTGCCACGTCCGCCGAGTAACGT-3′ |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sarapusit, S.; Lertkiatmongkol, P.; Duangkaew, P.; Rongnoparut, P. Modeling of Anopheles minimus Mosquito NADPH-Cytochrome P450 Oxidoreductase (CYPOR) and Mutagenesis Analysis. Int. J. Mol. Sci. 2013, 14, 1788-1801. https://doi.org/10.3390/ijms14011788
Sarapusit S, Lertkiatmongkol P, Duangkaew P, Rongnoparut P. Modeling of Anopheles minimus Mosquito NADPH-Cytochrome P450 Oxidoreductase (CYPOR) and Mutagenesis Analysis. International Journal of Molecular Sciences. 2013; 14(1):1788-1801. https://doi.org/10.3390/ijms14011788
Chicago/Turabian StyleSarapusit, Songklod, Panida Lertkiatmongkol, Panida Duangkaew, and Pornpimol Rongnoparut. 2013. "Modeling of Anopheles minimus Mosquito NADPH-Cytochrome P450 Oxidoreductase (CYPOR) and Mutagenesis Analysis" International Journal of Molecular Sciences 14, no. 1: 1788-1801. https://doi.org/10.3390/ijms14011788
APA StyleSarapusit, S., Lertkiatmongkol, P., Duangkaew, P., & Rongnoparut, P. (2013). Modeling of Anopheles minimus Mosquito NADPH-Cytochrome P450 Oxidoreductase (CYPOR) and Mutagenesis Analysis. International Journal of Molecular Sciences, 14(1), 1788-1801. https://doi.org/10.3390/ijms14011788