The Combination of Catechin and Epicatechin Gallate from Fructus Crataegi Potentiates β-Lactam Antibiotics Against Methicillin-Resistant Staphylococcus aureus (MRSA) in Vitro and in Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Combination of C and ECg Produces the Greatest ILSMR Effect in a Screening of Constituents from a Bioactive Fraction of Hawthorn
2.2. C and ECg Alone Have No ILSMR Effect, But When Combined, They Potentiate Antibacterial Effects of Oxacillin Against WHO-2
2.3. C in Combination with ECg Specifically Potentiate the Antibacterial Effects of β-Lactam Antibiotics Against Clinical MRSA Strains
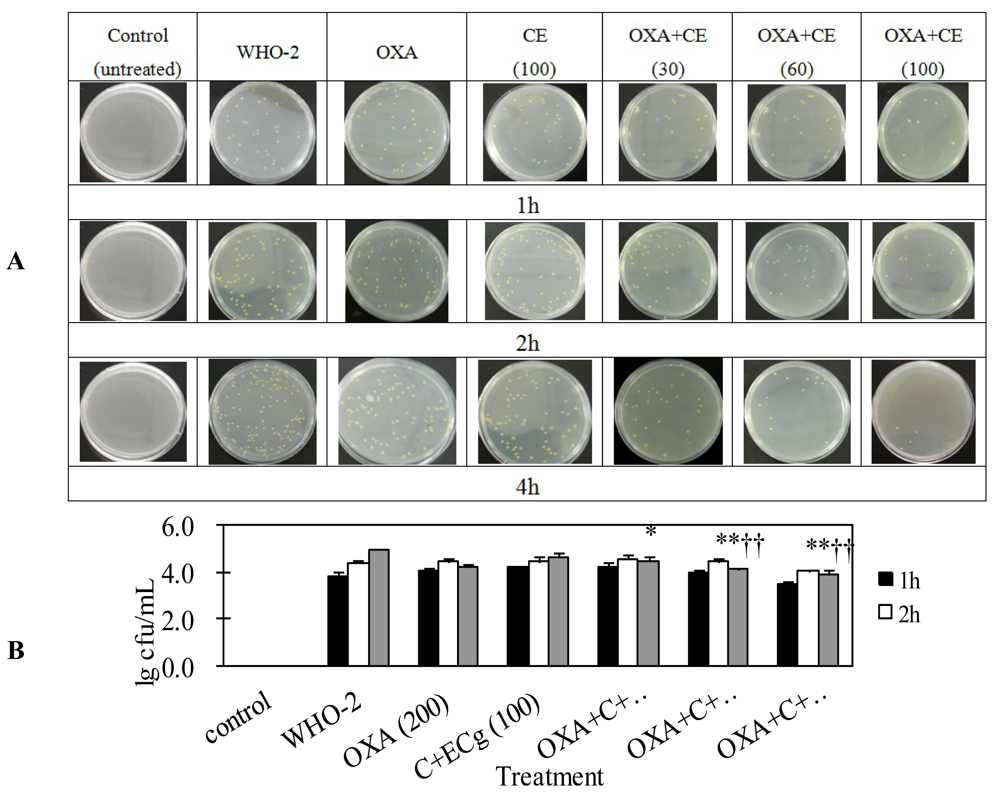
2.4. C and ECg in Combination with Oxacillin Markedly Decrease Whole Blood Bacterial Load Than Oxacillin Alone in Mice Challenged with Sublethal WHO-2
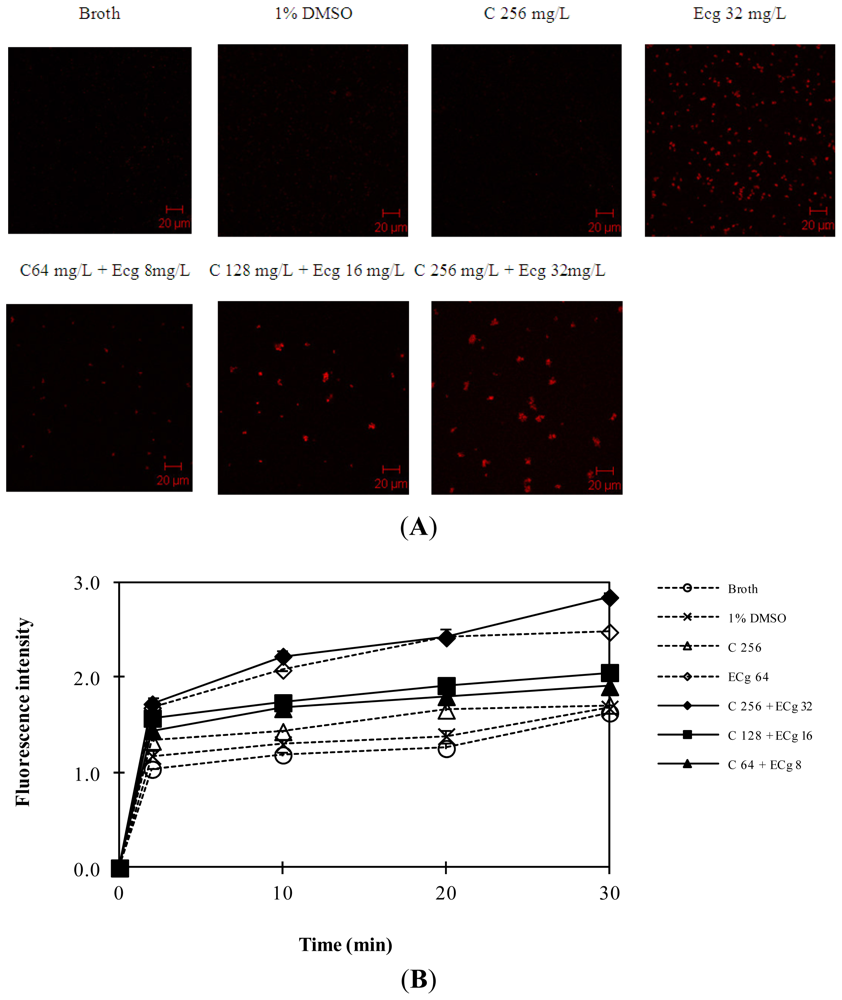
2.5. C in Combination with ECg Increases Accumulation of Daunorubicin within WHO-2
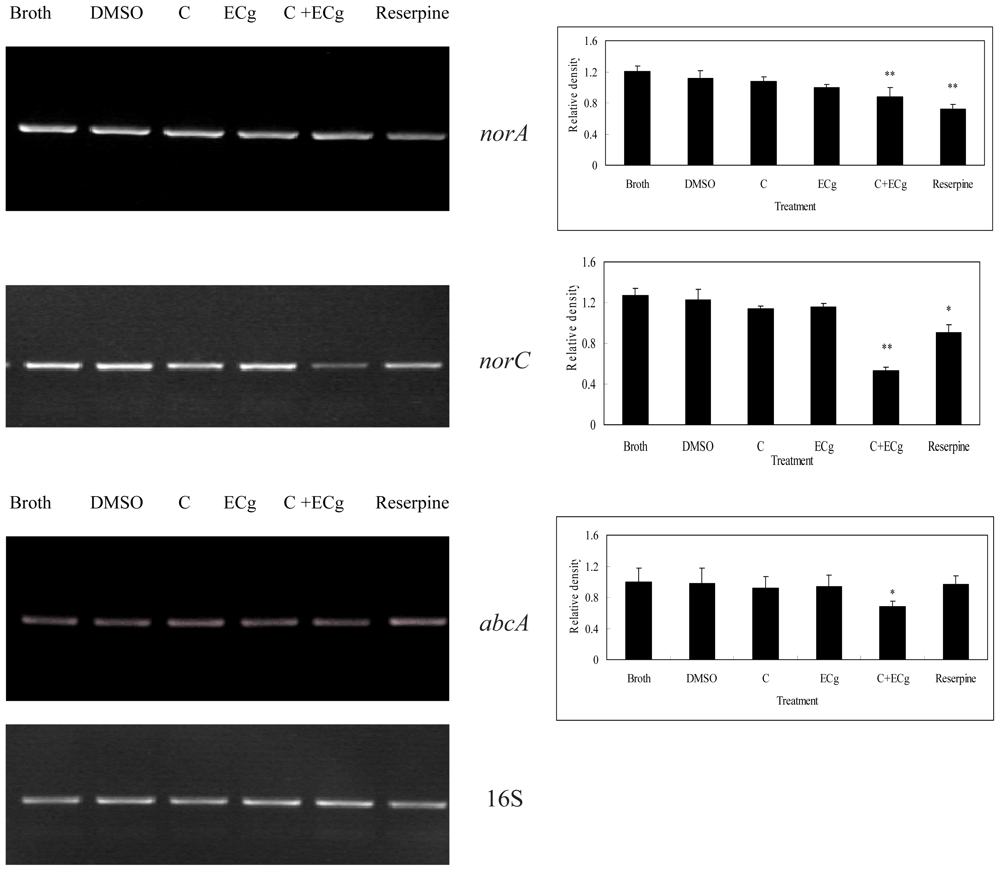
2.6. C in Combination with ECg DownRegulates mRNA Expressions of Efflux Pumps within WHO-2
2.7. Discussion
3. Experimental Section
3.1. Materials
3.2. Plant Material
3.3. Bacterial Strains
3.4. Animals
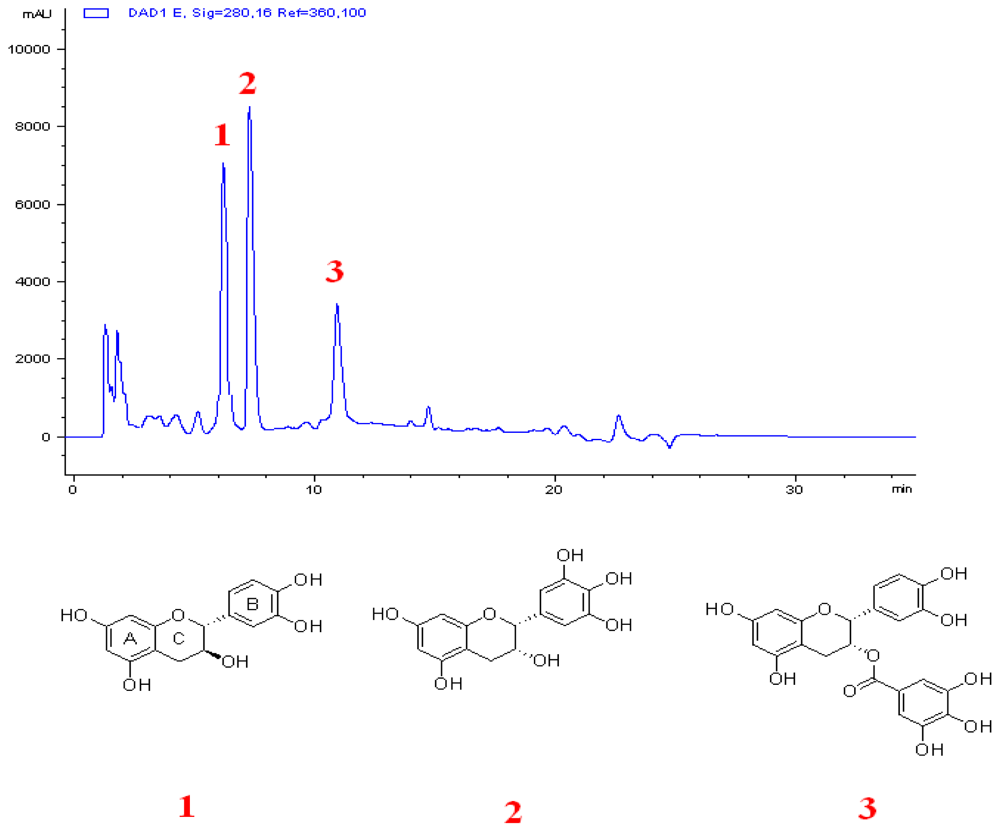
3.5. Extraction and Isolation
3.6. Bacterial Growth
3.7. Drug Susceptibility Assay
3.8. Experimental Animal Model and Drug Treatment
3.9. Accumulation of Daunorubicin within WHO-2
3.10. mRNA Expression Assays
3.11. Statistical Analysis and Presentation of Data
4. Conclusions
Supplementary Information
ijms-14-01802-s001.pdfAcknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Moellering, R.C., Jr. MRSA: The first half century. J. Antimicrob. Chemother. 2012, 67, 4–11. [Google Scholar]
- Livermore, D.M. Antibiotic resistance in Staphylococci. Int. J. Antimicrob. Agents 2000, 16, S3–S10. [Google Scholar]
- Pantosti, A.; Sanchinim, A.; Monaco, M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol 2007, 2, 323–334. [Google Scholar]
- Li, X.Z.; Nikaido, H. Efflux-Mediated drug resistance in bacteria: An update. Drugs 2009, 69, 1555–1623. [Google Scholar]
- Poole, K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med 2007, 39, 162–176. [Google Scholar]
- Taylor, P.W.; Stapleton, P.D.; Paul, L.J. New ways to treat bacterial infections. Drug Discov. Today 2002, 7, 1086–1091. [Google Scholar]
- Kondo, K.; Takaishi, Y.; Shibata, H.; Higuti, T. ILSMRs (intensifier of β-lactam-susceptibility in methicillin-resistant Staphylococcus aureus) from Tara [Caesalpinia spinosa (Molina) Kuntze]. Phytomedicine 2006, 13, 209–212. [Google Scholar]
- Liu, I.X.; Durham, D.G.; Richards, R.M. Baicalin synergy with beta-lactam antibiotics against methicillin-resistant Staphylococcus aureus and other beta-lactam-resistant strains of S. aureus. J. Pharm. Pharmacol 2000, 52, 361–366. [Google Scholar]
- Nicolson, K.; Evans, G.; O’Toole, P.W. Potentiation of methicillin activity against methicillin-resistant Staphylococcus aureus by diterpenes. FEMS Microbiol. Lett 1999, 179, 233–239. [Google Scholar]
- Shiota, S.; Shimizu, M.; Mizusima, T.; Ito, H.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Restoration of effectiveness of beta-lactams on methicillin-resistant Staphylococcus aureus by tellimagrandin I from rose red. FEMS Microbiol. Lett. 2000, 185, 135–138. [Google Scholar]
- Shimizu, M.; Shiota, S.; Mizushima, T.; Ito, H.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Marked potentiation of activity of beta-lactams against methicillin-resistant Staphylococcus aureus by corilagin. Antimicrob. Agents Chemother 2001, 45, 3198–3201. [Google Scholar]
- Eid, C.N.; Halligan, N.G.; Nicas, T.I.; Mullen, D.L.; Butler, T.F.; Loncharich, R.J.; Paschal, J.W.; Schofield, C.J.; Westwood, N.J.; Cheng, L. Tripeptide LY301621 and its diastereomers as methicillin potentiators against methicillin resistant Staphylococcus aureus. J. Antibiot (Tokyo) 1997, 50, 283–285. [Google Scholar]
- Hu, Z.Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother 2002, 46, 558–560. [Google Scholar]
- Shiota, S.; Shimizu, M.; Mizushima, T.; Ito, H.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Marked reduction in the minimum inhibitory concentration (MIC) of beta-lactams in methicillin-resistant Staphylococcus aureus produced by epicatechin gallate, an ingredient of green tea (Camellia sinensis). Biol. Pharm. Bull 1999, 22, 1388–1390. [Google Scholar]
- Stapleton, P.D.; Shah, S.; Anderson, J.C.; Hara, Y.; Hamilton-Miller, J.M.; Taylor, P.W. Modulation of beta-lactam resistance in Staphylococcus aureus by catechins and gallates. Int. J. Antimicrob. Agents 2004, 23, 462–467. [Google Scholar]
- Taylor, P.W.; Hamilton-Miller, J.M.; Stapleton, P.D. Antimicrobial properties of green tea catechins. Food Sci. Technol. Bull 2005, 2, 71–81. [Google Scholar]
- Pittler, M.H.; Schmidt, K.; Ernst, E. Hawthorn extract for treating chronic heart failure: Meta-Analysis of randomized trials. Am. J. Med 2003, 114, 665–674. [Google Scholar]
- Schüssler, M.; Hölzl, J.; Fricke, U. Myocardial effects of flavonoids from Crataegus species. Arzneimittel-Forschung 1995, 45, 842–845. [Google Scholar]
- Schwinger, R.H.; Pietsch, M.; Frank, K.; Brixius, K. Crataegus. special extract WS 1442 increases force of contraction in human myocardium cAMP-independently. J. Cardiovasc. Pharmacol 2000, 35, 700–707. [Google Scholar]
- Quettier-Deleu, C.; Voiselle, G.; Fruchart, J.C.; Duriez, P.; Teissier, E.; Bailleul, F.; Vasseur, J.; Trotin, F. Hawthorn extracts inhibit LDL oxidation. Pharmazie 2003, 58, 577–581. [Google Scholar]
- Tadić, V.M.; Dobrić, S.; Marković, G.M.; Dordević, S.M.; Arsić, I.A.; Menković, N.R.; Stević, T. Anti-Inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J. Agric. Food Chem 2008, 56, 7700–7709. [Google Scholar]
- Liuji, C.; Xianqiang, Y.; Mingxiang, J. Research progress on pharmacokinetics of tea catechins. Cha Ye Ke Xue 2001, 21, 11–16. [Google Scholar]
- Schmitz, F.J.; Fluit, A.C.; Lückefahr, M.; Engler, B.; Hofmann, B.; Verhoef, J.; Heinz, H.P.; Hadding, U.; Jones, M.E. The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother 1998, 42, 807–810. [Google Scholar]
- Gibbons, S.; Udo, E.E. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother Res 2000, 14, 139–140. [Google Scholar]
- Chang, Q.; Zuo, Y.; Harrison, F.; Chow, M.S. Hawthorn-An overview of chemical, pharmacological and clinical studies. J. Clin. Pharmacol 2002, 42, 605–612. [Google Scholar]
- Hamilton-Miller, J.M.; Shah, S. Activity of the tea component epicatechin gallate and analogues against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother 2000, 46, 852–853. [Google Scholar]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother 2001, 45, 1737–1742. [Google Scholar]
- Stapleton, P.D.; Shah, S.; Hara, Y.; Taylor, P.W. Potentiation of catechin gallate-mediated sensitization of Staphylococcus aureus to oxacillin by nongalloylated catechins. Antimicrob. Agents Chemother 2006, 50, 752–755. [Google Scholar]
- Qidong, Y.; Guoqiang, L. Chiral Drugs: Research and Evaluation; Chemical Industry Press: Beijing, China, 2011. [Google Scholar]
- Stapleton, P.D.; Shah, S.; Ehlert, K.; Hara, Y.; Taylor, P.W. The beta-lactam-resistance modifier (−)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology 2007, 153, 2093–2103. [Google Scholar]
- Bernal, P.; Lemaire, S.; Pinho, M.G.; Mobashery, S.; Hinds, J.; Taylor, P.W. Insertion of epicatechin gallate into the cytoplasmic membrane of methicillin-resistant Staphylococcus aureus disrupts penicillin-binding protein (PBP) 2a-mediated beta-lactam resistance by delocalizing PBP2. J. Biol. Chem 2010, 285, 24055–24065. [Google Scholar]
- Kosmidis, C.; Schindler, B.D.; Jacinto, P.L.; Patel, D.; Bains, K.; Seo, S.M.; Kaatz, G.W. Expression of multidrug resistance efflux pump genes in clinical and environmental isolates of Staphylococcus aureus. Int. J. Antimicrob. Agents 2012, 40, 204–209. [Google Scholar]
- Truong-Bolduc, Q.C.; Hooper, D.C. The transcriptional regulators NorG and MgrA modulate resistance to both quinolones and beta-lactams in Staphylococcus aureus. J. Bacteriol 2007, 189, 2996–3005. [Google Scholar]
- DeMarco, C.E.; Cushing, L.A.; Frempong-Manso, E.; Seo, S.M.; Jaravaza, T.A.; Kaatz, G.W. Efflux-Related resistance to norfloxacin, dyes, and biocides in bloodstream isolates of Staphylococcus aureus. Antimicrob. Agents Chemother 2007, 51, 3235–3239. [Google Scholar]
- Gibbons, S.; Moser, E.; Kaatz, G.W. Catechin gallates inhibit multidrug resistance (MDR) in Staphylococcus aureus. Planta. Med 2004, 70, 1240–1242. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother 2003, 52, 1. [Google Scholar]
- Hou, Z.; Meng, J.R.; Zhao, J.R.; Hu, B.Q.; Liu, J.; Yan, X.J.; Jia, M.; Luo, X.X. Inhibition of beta-lactamase-mediated oxacillin resistance in Staphylococcus aureus by a deoxyribozyme. Acta Pharmacol. Sin 2007, 28, 1775–1782. [Google Scholar]
| C (mg/L) a | ECg (mg/L) b | MIC of OXA (mg/L) | FICI |
|---|---|---|---|
| 0 | 0 | 512 | cannot calculate |
| 8 | 512 | 1.625 | |
| 16 | 256 | 0.625 | |
| 32 | 128 | 0.500 | |
| 16 | 0 | 512 | <1.016 |
| 8 | 512 | <1.073 | |
| 16 | 256 | <0 636 | |
| 32 | 64 | <0.386 | |
| 32 | 0 | 512 | <1.031 |
| 8 | 512 | <1.094 | |
| 16 | 256 | <0.656 | |
| 32 | 64 | <0.406 | |
| 64 | 0 | 512 | <1.063 |
| 8 | 256 | <0.625 | |
| 16 | 128 | <0.438 | |
| 32 | 32 | <0.375 | |
| 128 | 0 | 512 | <1.125 |
| 8 | 128 | <0.438 | |
| 16 | 64 | <0.375 | |
| 32 | <4 | <0.383 | |
| 256 | 0 | 512 | <1.250 |
| 8 | 64 | <0.438 | |
| 16 | 16 | <0.406 | |
| 32 | <4 | <0.508 | |
| 512 | 0 | 256 | <1.000 |
| 8 | 16 | <0.531 | |
| 16 | <4 | <0.633 | |
| 32 | <4 | <0.758 | |
| C a | EGC b | ECg c | OXA d MIC (mg/L) | FICI |
|---|---|---|---|---|
| 16 | 16 | 8 | 256 | <0.641 |
| 16 | 128 | <0.453 | ||
| 32 | 32 | <0.391 | ||
| 32 | 8 | 256 | <0.703 | |
| 16 | 128 | <0.516 | ||
| 32 | 32 | <0.454 | ||
| 64 | 8 | 256 | <0.829 | |
| 16 | 128 | <0.641 | ||
| 32 | <4 | <0.524 | ||
| 32 | 16 | 8 | 256 | <0.656 |
| 16 | 128 | <0.469 | ||
| 32 | 16 | <0.375 | ||
| 32 | 8 | 256 | <0.719 | |
| 16 | 128 | <0.531 | ||
| 32 | 16 | <0.438 | ||
| 64 | 8 | 128 | <0.594 | |
| 16 | 64 | <0.531 | ||
| 32 | <4 | <0.535 | ||
| 64 | 16 | 8 | 128 | <0.438 |
| 16 | 128 | <0.475 | ||
| 32 | 16 | <0.375 | ||
| 32 | 8 | 128 | <0.469 | |
| 16 | 64 | <0.406 | ||
| 32 | <4 | <0.409 | ||
| 64 | 8 | 128 | <0.594 | |
| 16 | 32 | <0.468 | ||
| 32 | <4 | <0.534 | ||
| 128 | 16 | 8 | 128 | <0.500 |
| 16 | 32 | <0.438 | ||
| 32 | <4 | <0.441 | ||
| 32 | 8 | 128 | <0.375 | |
| 16 | 32 | <0.438 | ||
| 32 | <4 | <0.503 | ||
| 64 | 8 | 16 | <0.469 | |
| 16 | <4 | <0.316 | ||
| 32 | <4 | <0.628 | ||
| 256 | 16 | 8 | 64 | <0.500 |
| 16 | 16 | <0.469 | ||
| 32 | <4 | <0.568 | ||
| 32 | 8 | 64 | <0.563 | |
| 16 | 32 | <0.563 | ||
| 32 | 16 | <0.656 | ||
| 64 | 8 | <4 | <0.565 | |
| 16 | <4 | <0.628 | ||
| 32 | <4 | <0.753 | ||
| 512 | 16 | 8 | 32 | <0.688 |
| 16 | <4 | <0.671 | ||
| 32 | <4 | <0.815 | ||
| 32 | 8 | 16 | <0.719 | |
| 16 | <4 | <0.754 | ||
| 32 | <4 | <0.878 | ||
| 64 | 8 | <4 | <0.815 | |
| 16 | <4 | <0.878 | ||
| 32 | <4 | <1.003 | ||
| Antibiotics | MIC (mg/L) of β-lactam antibiotics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration of C a + ECg b (mg/L) | |||||||||||
| 0 | 16 + 2 | 32 + 4 | |||||||||
| Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | FICI c | Range | MIC50 | MIC90 | FICI c | |
| oxacillin | 128–512 | 256 | 512 | 4–512 | 128 | 256 | 0.56 ± 0.32 (13/45) | 4–256 | 32 | 256 | 0.31 ± 0.22 (36/45) |
| ampicillin | 32–512 | 64 | 128 | 2–256 | 16 | 64 | 0.37 ± 0.26 (30/45) | 2–256 | 8 | 32 | 0.29 ± 0.24 (39/45) |
| ampicillin/sulbactam | 8–32 | 32 | 32 | 2–32 | 16 | 32 | 0.58 ± 0.29 (14/45) | 2–16 | 8 | 16 | 0.39 ± 0.23 (33/45) |
| cefazolin | 4–512 | 256 | 512 | 4–512 | 128 | 256 | 0.47 ± 0.33 (20/45) | 4–256 | 32 | 128 | 0.25 ± 0.18 (39/45) |
| cefepime | 8–2048 | 1024 | 1024 | 8–1024 | 256 | 512 | 0.45 ± 0.23 (18/45) | 8–1024 | 128 | 512 | 0.30 ± 0.20 (39/45) |
| imipenem/cilastatin | 4–512 | 128 | 256 | 4–512 | 32 | 64 | 0.36 ± 0.25 (29/45) | 4–512 | 4 | 32 | 0.23 ± 0.25 (41/45) |
| Antibiotics | MIC (mg/L) of β-lactam antibiotics | ||||||||||
| Concentration of Ca+ ECgb(mg/L) | |||||||||||
| 64 + 8 | 128 + 16 | ||||||||||
| Range | MIC50 | MIC90 | FICIc | Range | MIC50 | MIC90 | FICIc | ||||
| oxacillin | 4–256 | 4 | 128 | 0.28 ± 0.19 (42/45) | 4–256 | 4 | 4 | 0.41 ± 0.22 (31/45) | |||
| ampicillin | 2–256 | 2 | 16 | 0.27 ± 0.19 (42/45) | 2–256 | 2 | 2 | 0.29 ± 0.22 (31/45) | |||
| ampicillin/sulbactam | 2–16 | 2 | 4 | 0.30 ± 0.19 (42/45) | 2–16 | 2 | 2 | 0.44 ± 0.12 (33/45) | |||
| cefazolin | 4–64 | 4 | 32 | 0.23 ± 0.15 (44/45) | 4–4 | 4 | 4 | 0.39 ± 0.21 (32/45) | |||
| cefepime | 8–256 | 8 | 64 | 0.25 ± 0.17 (44/45) | 8–16 | 8 | 8 | 0.39 ± 0.23 (32/45) | |||
| imipenem/cilastatin | 4–512 | 4 | 4 | 0.28 ± 0.30 (42/45) | 4–512 | 4 | 4 | 0.45 ± 0.26 (32/45) | |||
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qin, R.; Xiao, K.; Li, B.; Jiang, W.; Peng, W.; Zheng, J.; Zhou, H. The Combination of Catechin and Epicatechin Gallate from Fructus Crataegi Potentiates β-Lactam Antibiotics Against Methicillin-Resistant Staphylococcus aureus (MRSA) in Vitro and in Vivo. Int. J. Mol. Sci. 2013, 14, 1802-1821. https://doi.org/10.3390/ijms14011802
Qin R, Xiao K, Li B, Jiang W, Peng W, Zheng J, Zhou H. The Combination of Catechin and Epicatechin Gallate from Fructus Crataegi Potentiates β-Lactam Antibiotics Against Methicillin-Resistant Staphylococcus aureus (MRSA) in Vitro and in Vivo. International Journal of Molecular Sciences. 2013; 14(1):1802-1821. https://doi.org/10.3390/ijms14011802
Chicago/Turabian StyleQin, Rongxin, Kangkang Xiao, Bin Li, Weiwei Jiang, Wei Peng, Jiang Zheng, and Hong Zhou. 2013. "The Combination of Catechin and Epicatechin Gallate from Fructus Crataegi Potentiates β-Lactam Antibiotics Against Methicillin-Resistant Staphylococcus aureus (MRSA) in Vitro and in Vivo" International Journal of Molecular Sciences 14, no. 1: 1802-1821. https://doi.org/10.3390/ijms14011802




