Structural Features of Alkaline Extracted Polysaccharide from the Seeds of Plantago asiatica L. and Its Rheological Properties
Abstract
:1. Introduction
2. Results
2.1. Structural Characterization
2.2. Rheological Properties of PLAP
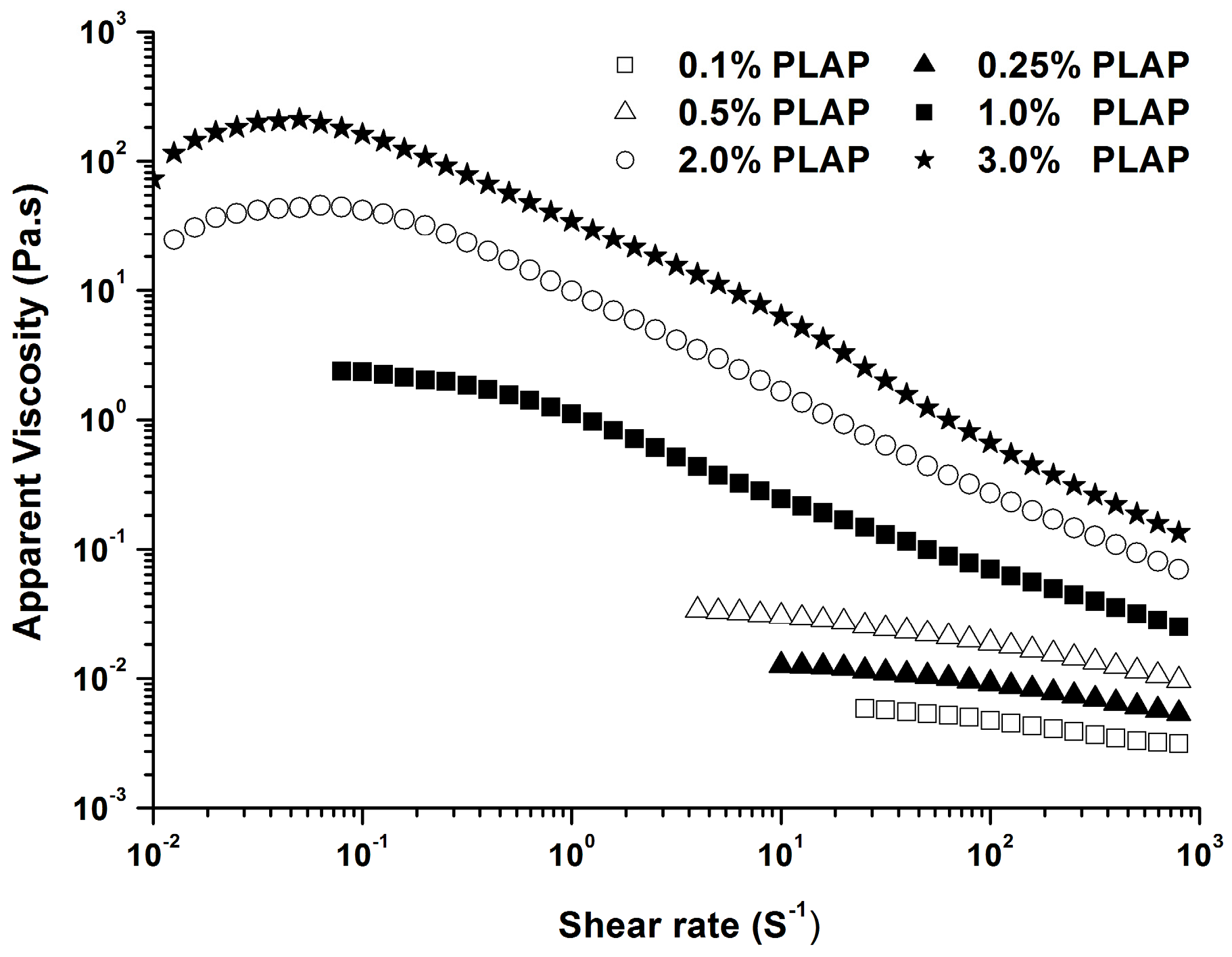
2.2.1. Steady State Shear Properties
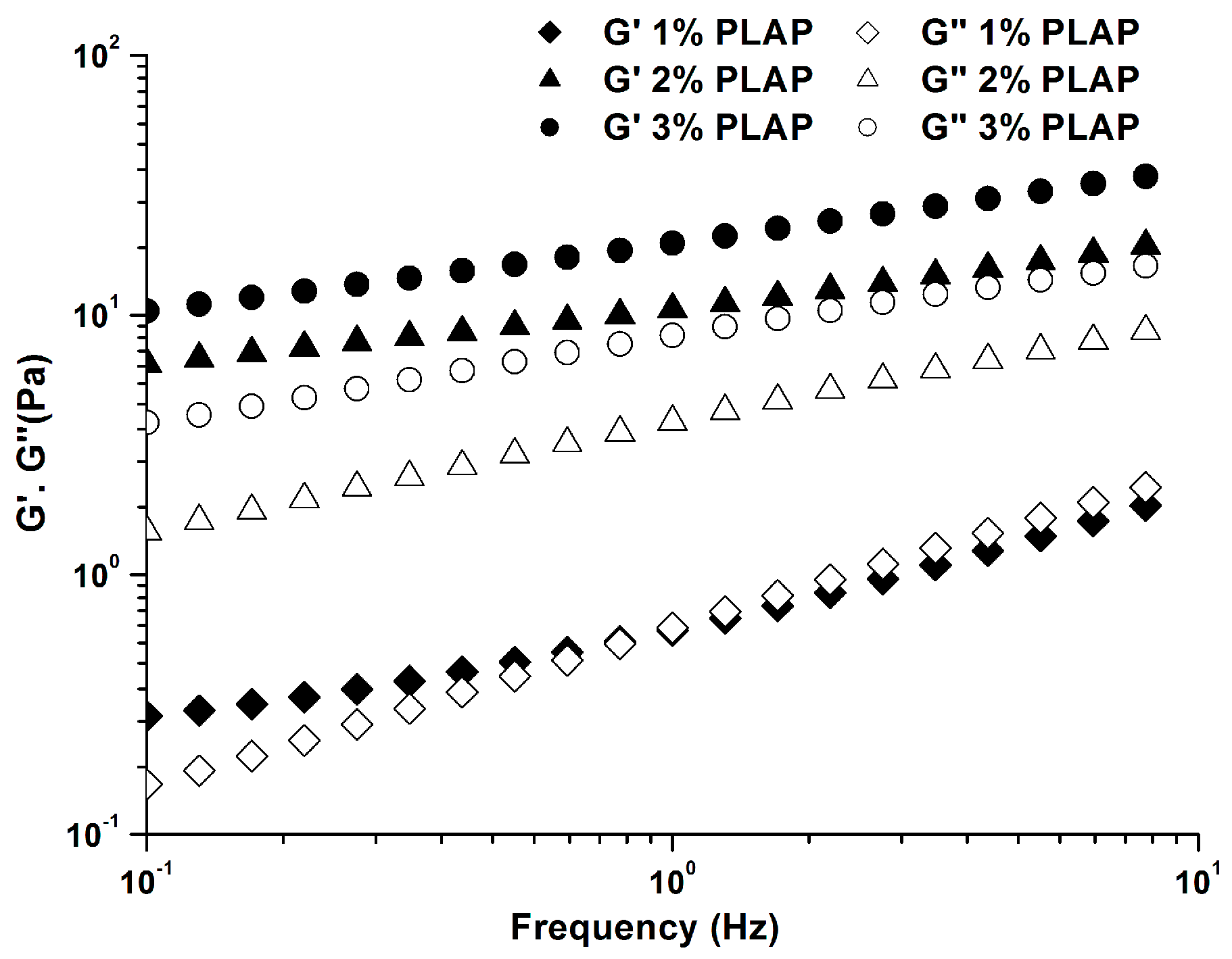
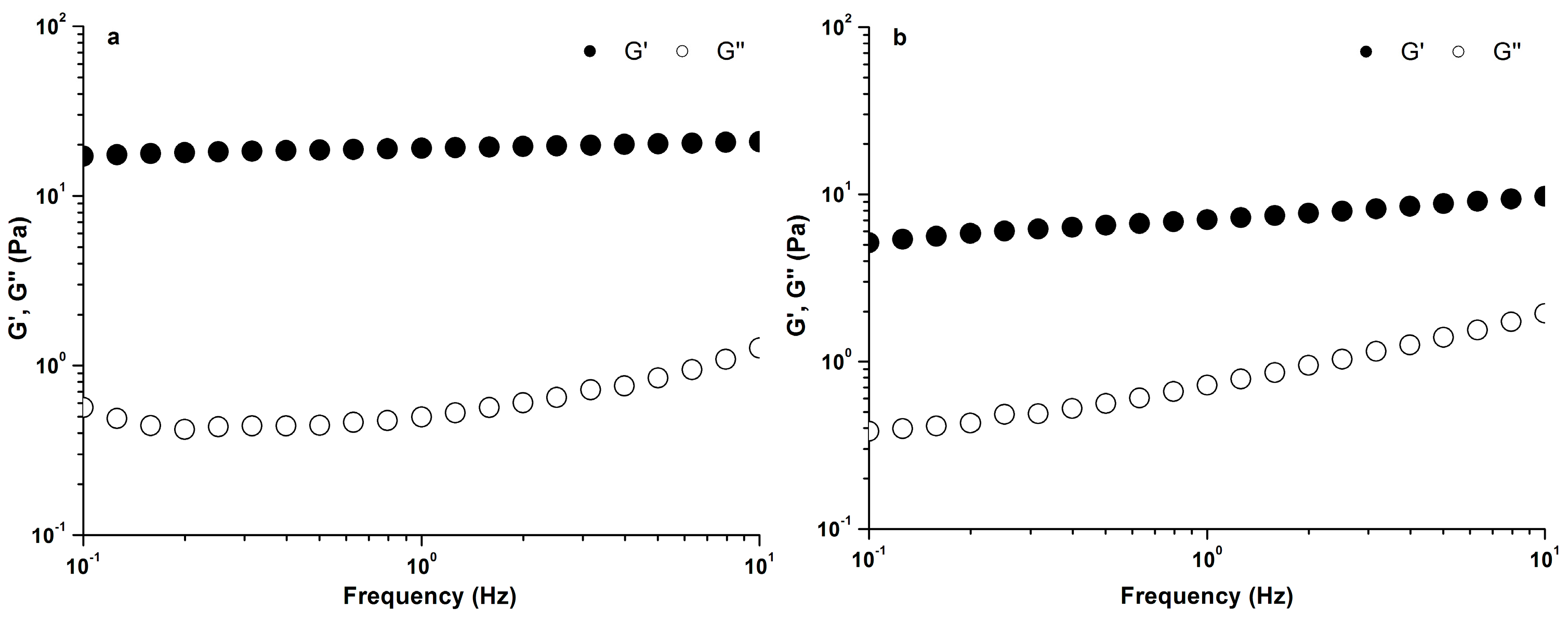
2.2.2. Viscoelastic Properties
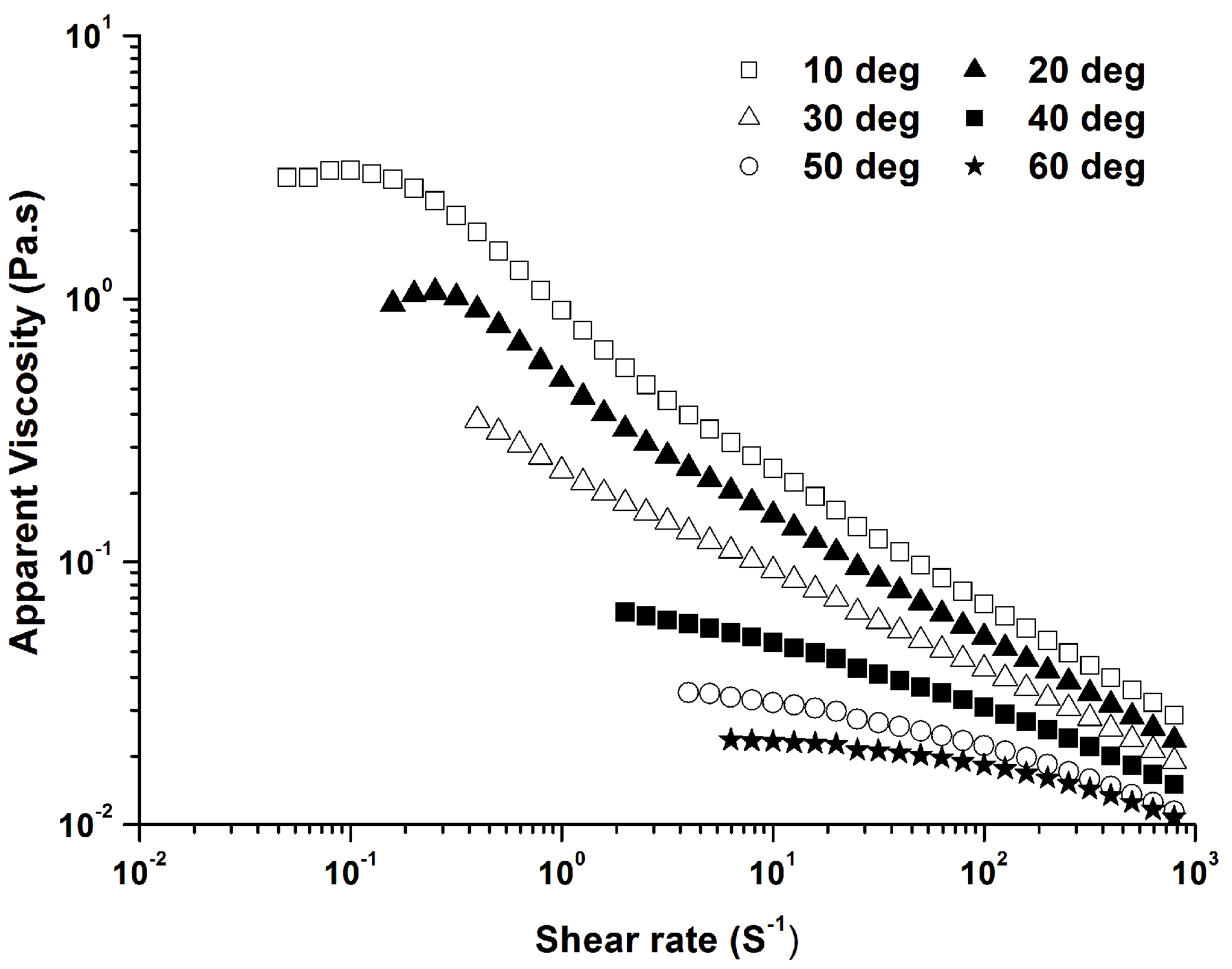
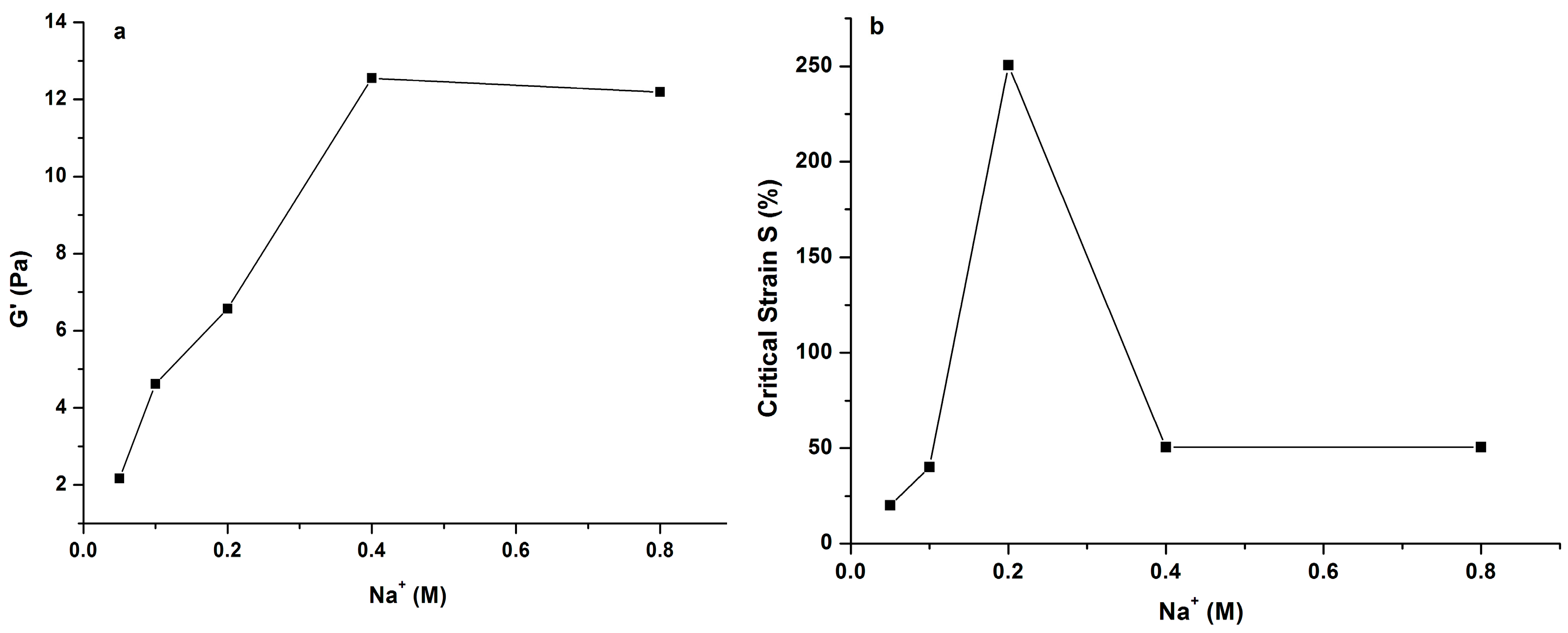
2.3. Effects of Na+ and Ca2+ on Rheological Properties of PLAP
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Polysaccharide Preparation
4.3. Physicochemical Characteristics
4.4. Methylation Analysis
4.5. Rheological Measurements
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. 2011, 51, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Ingerslev, A.K.; Theil, P.K.; Hedemann, M.S.; Laerke, H.N.; Knudsen, K.E. Resistant starch and arabinoxylan augment SCFA absorption, but affect postprandial glucose and insulin responses differently. Br. J. Nutr. 2014, 111, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.M.; Titgemeier, B.; Kirkpatrick, K.; Golubic, M.; Roizen, M.F. Major cereal grain fibers and psyllium in relation to cardiovascular health. Nutrients 2003, 5, 1471–1487. [Google Scholar] [CrossRef] [PubMed]
- Savitha Prashanth, M.R.; Shruthi, R.R.; Muralikrishna, G. Immunomodulatory activity of purified arabinoxylans from finger millet (Eleusine coracana, v. Indaf 15) bran. J. Food Sci. Technol. 2015, 52, 6049–6054. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, A.; Valentín, J.; Fernández, L.; Hernández-Jiménez, E.; López-Collazo, E.; Zerbes, P.; Schwörer, E.; Nuñéz, F.; Martín, I.G.; Sallis, H.; et al. Arabinoxylan rice bran (MGN-3/Biobran) enhances natural killer cell-mediated cytotoxicity against neuroblastoma in vitro and in vivo. Cytotherapy 2015, 17, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Al-Assaf, S.; Phillips, G.O.; Williams, P.A.; Takigami, S.; Dettmar, P.; Havler, M. Molecular weight, tertiary structure, water binding and colon behaviour of ispaghula husk fibre. Proc. Nutr. Soc. 2003, 62, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Cui, S.W.; Wang, Q.; Christopher Young, J. Fractionation and physicochemical characterization of psyllium gum. Carbohydr. Polym. 2008, 73, 35–43. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Sandhu, J.S.; Southgate, D.A. Structural data for the carbohydrate of ispaghula husk ex Plantago ovata forsk. Carbohydr. Res. 1979, 75, 265–274. [Google Scholar] [CrossRef]
- Pawar, H.; Varkhade, C. Isolation, characterization and investigation of Plantago ovata husk polysaccharide as superdisintegrant. Int. J. Biol. Macromol. 2014, 69, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Q.; Sun, Y.; Yang, B.; Wang, Z.; Chai, G.; Guan, Y.; Zhu, W.; Shu, Z.; Lei, X.; et al. Purification, characterization and immunomodulatory effects of Plantago depressa polysaccharides. Carbohydr. Polym. 2014, 112, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Gonҫalves, S.; Romano, A. The medicinal potential of plants from the genus Plantago (Plantaginaceae). Ind. Crop. Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- Farahnaky, A.; Askari, H.; Majzoobi, M.; Mesbahi, G. The impact of concentration, temperature and pH on dynamic rheology of psyllium gels. J. Food Eng. 2010, 100, 294–301. [Google Scholar] [CrossRef]
- Guo, Q.; Cui, S.W.; Wang, Q.; Goff, H.D.; Smith, A. Microstructure and rheological properties of psyllium polysaccharide gel. Food Hydrocoll. 2009, 23, 1542–1547. [Google Scholar] [CrossRef]
- Haque, A.; Richardson, R.K.; Morris, E.R.; Dea, I. Anthan-like ‘weak gel’ rheology from dispersions of ispaghula seed husk. Carbohydr. Polym. 1993, 22, 223–232. [Google Scholar] [CrossRef]
- Yin, J.Y.; Lin, H.; Li, J.; Wang, Y.; Cui, S.W.; Nie, S.P.; Xie, M.Y. Structural characterization of a highly branched polysaccharide from the seeds of Plantago asiatica L. Carbohydr. Polym. 2012, 87, 2416–2424. [Google Scholar] [CrossRef]
- Yin, J.Y.; Lin, H.X.; Nie, S.P.; Cui, S.W.; Xie, M.Y. Methylation and 2D NMR analysis of arabinoxylan from the seeds of Plantago asiatica L. Carbohydr. Polym. 2012, 88, 1395–1401. [Google Scholar] [CrossRef]
- Yin, J.Y.; Nie, S.P.; Li, J.; Li, C.; Cui, S.W.; Xie, M.Y. Mechanism of interactions between calcium and viscous polysaccharide from the seeds of Plantago asiatica L. J. Agric. Food Chem. 2012, 60, 7981–7987. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Nie, S.P.; Min, F.F.; Xie, M.Y. Polysaccharide from seeds of Plantago asiatica L. increases short-chain fatty acid production and fecal moisture along with lowering pH in mouse colon. J. Agric. Food Chem. 2012, 60, 11525–11532. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Nie, S.P.; Li, C.; Fu, Z.H.; Xie, M.Y. Microbial short-chain fatty acid production and extracellular enzymes activities during in vitro fermentation of polysaccharides from the seeds of Plantago asiatica L. treated with microwave irradiation. J. Agric. Food Chem. 2013, 61, 6092–6101. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Nie, S.P.; Wu, Q.M.; Li, C.; Fu, Z.H.; Gong, J.; Cui, S.W.; Xie, M.Y. Polysaccharide from seeds of Plantago asiatica L. affects lipid metabolism and colon microbiota of mouse. J. Agric. Food Chem. 2014, 62, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.S.; Pai, D.A.; Hamaker, B.R.; Campanella, O.H. Structure–function relationships for corn bran arabinoxylans. J. Cereal Sci. 2010, 52, 368–372. [Google Scholar] [CrossRef]
- Skendi, A.; Biliaderis, C.; Izydorczyk, M.; Zervou, M.; Zoumpoulakis, P. Structural variation and rheological properties of water-extractable arabinoxylans from six Greek wheat cultivars. Food Chem. 2011, 126, 526–536. [Google Scholar] [CrossRef]
- Clark, A.H.; Ross-Murphy, S.B. Structural and mechanical properties of biopolymer gels. Biopolymers 1987, 83, 57–192. [Google Scholar]
- Rincón, F.; Muñoz, J.; León de Pinto, G.; Alfaro, M.; Calero, N. Rheological properties of Cedrela odorata gum exudate aqueous dispersions. Food Hydrocoll. 2009, 23, 1031–1037. [Google Scholar] [CrossRef]
- Simas-Tosin, F.; Barraza, R.; Petkowicz, C.; ilveira, J.; Sassaki, G.; Santos, E.; Gorin, P.; Iacomini, M. Rheological and structural characteristics of peach tree gum exudate. Food Hydrocoll. 2010, 24, 486–493. [Google Scholar] [CrossRef]
- Fischer, M.H.; Yu, N.; Gray, G.R.; Ralph, J.; Anderson, L.; Marlett, J.A. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk). Carbohydr. Res. 2004, 339, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhang, H.; Niu, Y.; Chen, L.; Liu, J.; Alaxi, S.; Shang, P.; Yu, W.; Yu, L. A novel alkali extractable polysaccharide from Plantago asiatic L. seeds and its radical-scavenging and bile acid-binding activities. J. Agric. Food Chem. 2015, 63, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, A.; Lund, I.; Djahromi, J.; Paulsen, B.; Wold, J.; Knutsen, S. Structural features and anti-complementary activity of some heteroxylan polysaccharide fractions from the seeds of Plantago major L. Carbohydr. Polym. 1999, 38, 133–143. [Google Scholar] [CrossRef]
- Yin, J.Y.; Nie, S.P.; Guo, Q.B.; Wang, Q.; Cui, S.W.; Xie, M.Y. Effect of calcium on solution and conformational characteristics of polysaccharide from seeds of Plantago asiatica L. Carbohydr. Polym. 2015, 124, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Rattan, O.; Izydorczyk, M.S.; Biliaderis, C.G. Structure and rheological behaviour of arabinoxylans from Canadian bread wheat flours. LWT-Food Sci. Technol. 1994, 27, 550–555. [Google Scholar] [CrossRef]
- Cui, W.; Mazza, G. Physicochemical characteristics of flaxseed gum. Food Res. Int. 1996, 29, 397–402. [Google Scholar] [CrossRef]
- Qian, K.; Cui, S.; Wu, Y.; Goff, H. Flaxseed gum from flaxseed hulls: Extraction, fractionation, and characterization. Food Hydrocoll. 2012, 28, 275–283. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, S.W. Understanding the physical properties of food polysaccharides. In Food Carbohydrates: Chemistry, Physical Properties, and Applications; Cui, S.W., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Staub, A. Removal of protein-Sevag method. Methods Carbohydr. Chem. 1965, 5, 5–6. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhou, C.; Xie, M.Y.; Wan, Y.; Nie, S.P. Study on determination of the contents of polysaccharides in Semen Plantaginis. Chin. J. Anal. Lab. 2008, 27, 10. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.Y.; Nie, S.P.; Li, C.; Wang, Y.X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Ciucanu, I.; Kerek, F.A. Simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Yin, J.Y.; Chan, B.C.L.; Yu, H.; Lau, I.Y.K.; Han, X.Q.; Cheng, S.W.; Wong, C.K.; Lau, C.B.S.; Xie, M.Y.; Fung, K.P. Separation, structure characterization, conformation and immunomodulating effect of a hyperbranched heteroglycan from Radix Astragali. Carbohydr. Polym. 2012, 87, 667–675. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds PLAP are available from the authors.


| Sugar (%) | Protein (%) | Uronic Acid (%) | (η) (dL/g) a | Molecular Weight, Mw (×10−6) b | Monosaccharide Composition (Molar Ratio) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Rha | Ara | Xyl | Glc | Gal | |||||
| 82.84 | 0.68 | 20.50 | 5.81 | 3.80 | 1.00 | 15.41 | 63.95 | 1.29 | 2.58 |
| Residue Linkage | Molar Ratio a | m/z |
|---|---|---|
| T-linked Araf | 5.06 | 43, 71, 87, 101, 102, 118, 129, 161 |
| 1,3-linked Araf | 10.22 | 43, 69, 87, 99, 113, 118, 129, 233 |
| T-linked Xylp | 10.88 | 43, 59, 87, 88, 101, 102, 117, 118, 161, 162 |
| 1,3-linked Xylp | 11.39 | 43, 59, 87, 101, 117, 118, 129, 173, 174 |
| 1,4-linked Xylp | 6.19 | 43, 87, 99, 102, 118, 129, 162, 189 |
| 1,2,4-linked Xylp | 13.30 | 43, 57, 71, 87, 88, 129, 130, 189, 190 |
| 1,3,4-linked Xylp | 38.98 | 43, 85, 87, 99, 118, 201, 261 |
| T-linked Galp | 1.12 | 43, 59, 71, 87, 101, 102, 118, 129, 145, 161, 162, 205 |
| 1,4-linked Galp | 1.19 | 43, 87, 99, 102, 113, 118, 129, 162, 173, 233 |
| 1,2-linked Rhap | 0.65 | 43, 88, 89, 100, 101, 115, 130, 131, 161, 190 |
| 1,3-linked Glcp | 0.40 | 43, 71, 87, 101, 118, 129, 161, 234 |
| Concentration (wt %) | η0 a (Pa.s) | α b (s) | m c |
|---|---|---|---|
| 0.10 | 0.0104 | 0.0174 | 0.3402 |
| 0.25 | 0.0160 | 0.0056 | 0.4732 |
| 0.50 | 0.0448 | 0.0204 | 0.4615 |
| 1.0 | 1.8271 | 1.3301 | 0.5003 |
| 2.0 | 44.0297 | 4.4526 | 0.8284 |
| 3.0 | 300.2550 | 9.4121 | 0.8788 |
| Temperature | η0 a (Pa.s) | α b (s) | m c |
|---|---|---|---|
| 10 | 7.8691 | 34.1772 | 0.4173 |
| 20 | 2.4583 | 15.1757 | 0.4755 |
| 30 | 0.5766 | 3.3620 | 0.5506 |
| 40 | 0.0966 | 0.0917 | 0.5945 |
| 50 | 0.0392 | 0.0094 | 0.5508 |
| 60 | 0.0226 | 0.0015 | 0.4250 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.-Y.; Chen, H.-H.; Lin, H.-X.; Xie, M.-Y.; Nie, S.-P. Structural Features of Alkaline Extracted Polysaccharide from the Seeds of Plantago asiatica L. and Its Rheological Properties. Molecules 2016, 21, 1181. https://doi.org/10.3390/molecules21091181
Yin J-Y, Chen H-H, Lin H-X, Xie M-Y, Nie S-P. Structural Features of Alkaline Extracted Polysaccharide from the Seeds of Plantago asiatica L. and Its Rheological Properties. Molecules. 2016; 21(9):1181. https://doi.org/10.3390/molecules21091181
Chicago/Turabian StyleYin, Jun-Yi, Hai-Hong Chen, Hui-Xia Lin, Ming-Yong Xie, and Shao-Ping Nie. 2016. "Structural Features of Alkaline Extracted Polysaccharide from the Seeds of Plantago asiatica L. and Its Rheological Properties" Molecules 21, no. 9: 1181. https://doi.org/10.3390/molecules21091181
APA StyleYin, J.-Y., Chen, H.-H., Lin, H.-X., Xie, M.-Y., & Nie, S.-P. (2016). Structural Features of Alkaline Extracted Polysaccharide from the Seeds of Plantago asiatica L. and Its Rheological Properties. Molecules, 21(9), 1181. https://doi.org/10.3390/molecules21091181