3.1. Chemistry
Melting points are uncorrected. NMR spectra were recorded using a Bruker Ascend 600 spectrometer (Bruker, Billerica, MS, USA). To assign the structures, the following 2D experiments were employed: 1H-13C gradient selected HSQC (Heteronuclear Single Quantum Coherence) and HMBC (Heteronuclear Multiple Bond Coherence) sequences. Standard experimental conditions and standard Bruker program were used. The 1H- and 13C-NMR spectral data are given relative to the TMS signal at 0.0 ppm. EI MS spectra were recorded using an LKB GC MS 20091 spectrometer at 75 eV (LKB, Bromma, Sweden).
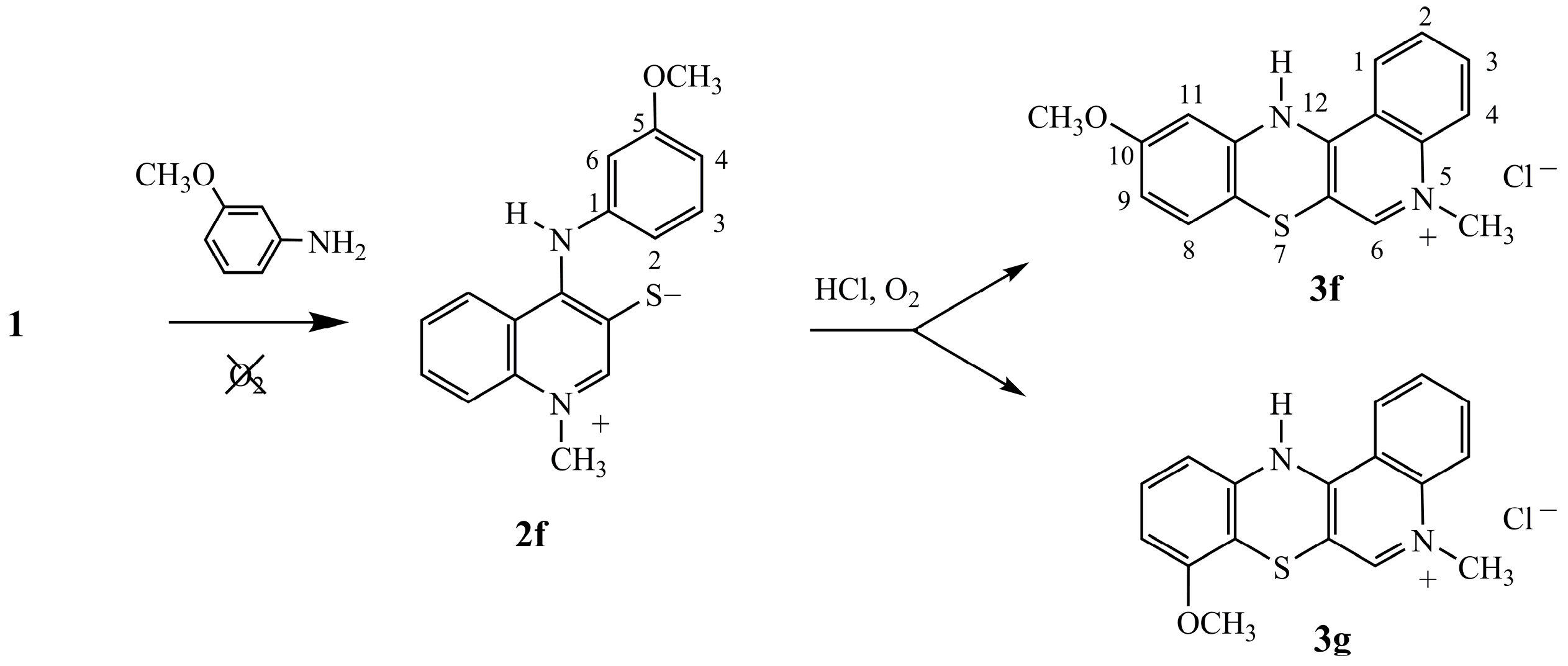
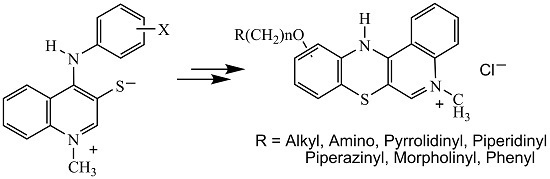
3.1.1. Synthesis of 1-Methyl-4-(arylamino)quinolinium-3-thiolates 2
Argon was passed through the suspension of bis-chloride (1) (0.419 g, 1 mmol) in dry pyridine (15 mL) at room temperature over 15 min. Amine (2.5 mmol) was added and the reaction mixture was bubbled with argon for a further 15 min. The mixture was then stirred at room temperature for 7 days. The solid product was filtered off and washed with dry ether. The raw product was purified through recrystallization from ethanol.
1-Methyl-4-((4-methoxy)phenylamino)quinolinium-3-thiolate (2a). Yield: 54%; m.p. 173 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.77 (s, 3H, OCH3), 4.16 (s, 3H, NCH3), 6.89–6.98 (m, 2H, Harom), 7.06–7.13 (m, 2H, Harom), 7.20–7.28 (m, 1H, H6quinolinyl), 7.42–7.48 (m, 1H, H8quinolinyl), 7.55–7.62 (m, 1H, H7quinolinyl), 7.88–7.94 (m, 1H, H5quinolinyl), 8.70 (s, 1H, H2quinolinyl), 9.99 (s, 1H, NH); 13C-NMR (DMSOd-6, 150.9 MHz) δ (ppm): 42.26 (NCH3), 55.79 (OCH3), 115.20 (C2′, C6′), 118.90 (C5), 124.54 (C4a), 124.65 (C8), 124.90 (C6), 124.99 (C3′, C5′), 129.57 (C7), 134.71 (C3), 135.74 (C8a), 145.00 (C2), 151.16 (C4′), 153.43 (C4), 157.47 (C1′); EI-MS (70eV) (m/z): 296 (M+, 100%); Anal. calcd. for C17H16N2OS: C, 68.89; H, 5.44; N, 9.45; S, 10.82. Found: C, 68.81; H, 5.35; N, 9.40; S, 8.78.
1-Methyl-4-((2-methoxy)phenylamino)quinolinium-3-thiolate (2b). Yield: 58%; m.p. 172–174 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.77 (s, 3H, OCH3), 4.19 (s, 3H, NCH3), 6.84–6.93 (m, 2H, Harom), 7.12–7.22 (m, 2H, Harom), 7.25–7.31 (m, 1H, H6quinolinyl), 7.45–7.49 (m, 1H, H8quinolinyl), 7.57–7.63 (m, 1H, H7quinolinyl), 7.89–7.94 (m, 1H, H5quinolinyl), 8.73 (s, 1H, H2quinolinyl), 9.86 (s, 1H, NH); EI-MS (70 eV) (m/z): 296 (M+, 100%); Anal. calcd. for C17H16N2OS: C, 68.89; H, 5.44; N, 9.45; S, 10.82. Found: C, 8.78; H, 5.39; N, 9.38; S, 8.75.
1-Methyl-4-((2-hydroxy-4-phenyl)phenylamino)quinolinium-3-thiolate (2c). Yield: 37%; m.p. 198 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 4.19 (s, 3H, NCH3), 7.03–7.08 (m, 1H, Harom), 7.15–7.18 (m, 1H, Harom), 7.22–7.26 (m, 1H, Harom), 7.28–7.37 (m, 3H, Harom), 7.37–7.40 (m, 1H, Harom), 7.40–7.48 (m, 2H, Harom), 7.60–7.67 (m, 1H, H7quinolinyl), 7.67–7.73 (m, 1H, H8quinolinyl), 7.93–7.98 (m, 1H, H5quinolinyl), 8.74 (s, 1H, H2quinolinyl), 9.97 (s, 1H, NH), 10.17 (s, 1H, OH), EI-MS (70 eV) (m/z): 358 (M+, 100%); Anal. calcd. for C22H18N2OS: C, 73.72; H, 5.06; N, 7.81; S, 8.94. Found: C, 73.67; H, 4.97; N, 7.76; S 8.91.
1-Methyl-4-(4-(N-piperidinyl)phenylamino)quinolinium-3-thiolate (2d). Yield: 84%; m.p. 208 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 1.45–1.80 (m, 6H, Hpiperidinyl), 3.10–3.30 (m, 4H, Hpiperidinyl), 4.15 (s, 3H NCH3), 7.03–7.08 (m, 4H, Harom), 7.25–7.38 (m, 1H, Harom), 7.55–7.74 (m, 1H, Harom), 7.92–7.98 (m, 1H, Harom), 8.69 (s, 1H, H2quinolinyl), 10.08 (s, 1H, NH); EI-MS (70 eV) (m/z): 349 (M+, 100%); Anal. calcd. for C21H23N3S: C, 72.17; H, 6.63; N, 12.02; S, 9.17. Found: C, 72.15; H, 6.54; N, 11.95; S, 9.13.
1-Methyl-4-(4-(N-piperazinyl)phenylamino)quinolinium-3-thiolate (2e). Yield: 86%; m.p. 137 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.05–3.35 (m, 8H, Hpiperazinyl), 4.17 (s, 3H, NCH3), 4.69 (s, 1H, NHpiperazinyl), 6.96–7.02 (m, 2H, Harom), 7.06–7.10 (m, 2H, Harom), 7.25–7.30 (m, 1H, Harom), 7.51–7.55 (m, 1H, Harom), 7.58–7.64 (m, 1H, Harom), 7.90–7.96 (m, 1H, Harom), 8.70 (s, 1H, H2quinolinyl), 10.01 (s, 1H, NH); EI-MS (70 eV) (m/z): 350 (M+, 100%); Anal. calcd. for C20H22N4S: C, 68.54; H, 6.33; N, 15.99; S, 9.15. Found: C, 68.49; H, 6.25; N, 15.90; S, 9.10.
1-Methyl-4-(3-methoxyphenylamino)quinolinium-3-thiolate (2f). Yield: 66%; m.p. 181 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.69 (s, 3H, OCH3), 4.20 (s, 3H, NCH3), 6.58–6.63 (m, 1H, Harom), 6.69–6.77 (m, 1H, Harom), 7.20–7.26 (m, 1H, H4arom), 7.29–7.35 (m, 1H, H6quinolinyl), 7.49–7.54 (m, 1H, H8quinolinyl), 7.58–7.64 (m, 1H, H7quinolinyl), 7.94–7.99 (m, 1H, H5quinolinyl), 8.80 (s, 1H, H2quinolinyl), 9.91 (s, 1H, NH); EI-MS (70 eV) (m/z): 296 (M+, 100%); Anal. calcd for C17H16N2OS: C 68.89; H 5.44; N 9.45; S 10.82. Found: C, 68.79; H, 5.36; N, 9.40; S, 10.75.
3.1.2. Synthesis of 5-Methyl-12(H)-quino[3,4-b][1,4]benzothiazinium Chloride 3
Procedure (A): Aniline hydrochloride (0.155 g, 1.2 mmol) was added to the mixture of 3-thiolate (2) (1 mmol) in 10 mL of dry pyridine and the whole was mixed at 70 °C for 12 h. After cooling it down to room temperature, the formed precipitate was filtered off and washed with ether. The raw product was recrystallized from ethanol.
Procedure (B): Amine (2.5 mmol) was added to the mixture of bis-chloride (1) (0.419 g, 1 mmol) in 10 mL of dry pyridine and the whole was mixed at 70 °C for 12 h. The mixture was cooled down to room temperature and the formed precipitate was filtered off and washed with ether. The raw product was purified through recrystallization from ethanol.
9-Methoxy-5-methyl-12(H)-quino[3,4-b][1,4]benzothiazinium chloride (3a). Yield: Procedure (A) 68%, Procedure (B) 72%; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.73 (s, 3H, OCH3), 4.19 (s, 3H, NCH3), 6.70–6.78 (m, 2H, H10, H11), 7.36–7.44 (m, 1H, H8), 7.76–7.84 (m, 1H, H2), 7.97–8.08 (m, 2H, H3, H4), 8.50–8.63 (m, 1H, H1), 8.84 (s, 1H, H6), 10.93 (s, 1H, NH); Anal. calcd. for C17H15ClN2OS: C, 61.72; H, 4.57; N, 8.47; S, 9.69. Found C, 61.63; H, 4.51; N, 8.41; S, 9.66.
11-Methoxy-5-methyl-12(H)-quino[3,4-b][1,4]benzothiazinium chloride (3b). Yield: Procedure (A) 65%, Procedure (B) 74%; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.93 (s, 3H, OCH3), 4.18 (s, 3H, NCH3), 6.68–6.77 (d, 3J = 9 Hz, 1H, H8), 6.96–7.04 (d, 3J = 7.8 Hz, H10), 7.08–7.16 (d.d, 3J = 9 Hz, 3J = 7.8 Hz, H9), 7.82–7.90 (s, 1H, H2). 8.03–8.16 (m, 2H, H3, H4), 8.50–8.58 (m, 1H, H1), 8.80 (s, 1H, H6), 9.90 (s, 1H, NH); 13C-NMR (DMSOd-6, 150.9 MHz) δ (ppm): 43.28 (NCH3), 56.94 (OCH3), 107.54 (C6a), 112.28 (C12b), 116.28 (C8), 118.83 (C12a), 119.28 (C11), 119.32 (C10), 123.88 (C2), 125.40 (C11a), 128.10 (C9), 128.47 (C1), 134.99 (C3), 139.11 (C6), 144,20 (C4a), 148.90 (C7a), 152.03 (C4); Anal. calcd. for C17H15ClN2OS: C, 61.72; H, 4.57; N, 8.47, S, 9.69. Found: C, 6.66; H, 4.49; N, 8.45; S, 9.64.
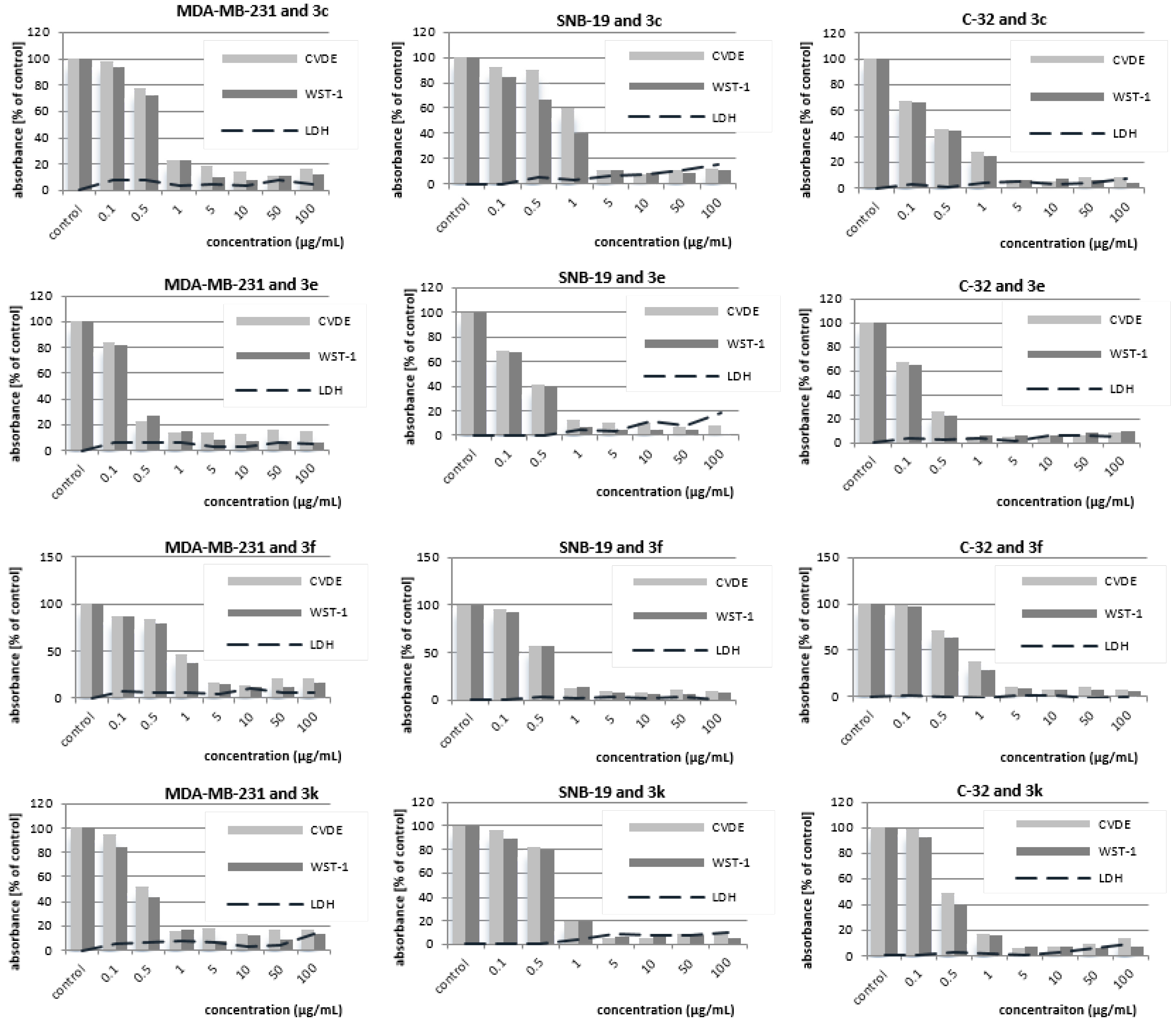
11-Hydroxy-9-phenyl-5-methyl-12(H)-quino[3,4-b][1,4]benzothiazinium chloride (3c). Yield: Procedure (A) 59%, Procedure (B) 65%; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 4.06 (s, 3H, NCH3), 6.93–7.02 (m, 2H, Harom), 7.30–7.35 (m, 2H, Harom), 7.38–7.44 (m, 1H, Harom), 7.44–7.51 (m, 2H, Harom), 7.74–7.85 (m, 1H, Harom), 7.98–8.07 (m, 2H, Harom), 8.60–8.65 (m, 2H, Harom), 10.10 (s, 1H, NH), 11.18 (s, 1H, OH); Anal. calcd. for C22H17ClN2OS: C, 67.25; H, 4.36; N, 7.13; S, 8.16. Found: C, 67.18; H, 4.30; N, 7.04; S 8.10.
9-(N-piperidinyl)-5-methyl-12(H)-quino[3,4-b][1,4]benzothiazinium chloride (3d). Yield: Procedure (A) 68%, Procedure (B) 77%; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 1.43–1.70 (m, 6H, Hpiperidinyl), 3.08–3.20 (m, 4H, Hpiperidinyl). 4.05 (s, 3H, NCH3), 6.56–6.62 (d, 4J = 2.4 Hz, 1H, H8), 6.64–6.69 (d.d, 3J = 9 Hz, 4J = 2.4 Hz, 1H, H10), 7.44–7.50 (d, 3J = 9 Hz, 1H, H11), 6.70–6.75 (m, 1H, H2), 7.74–7.79 (m, 2H, H3, H4), 8.50 (s, 1H, H6), 9.01–9.06 (m, 1H, H1), 11.22 (s, 1H, NH). Anal. calcd. for C21H22ClN3S: C, 65.70; H, 5.78; N, 10.94; S, 8.35. Found C, 65.64; H, 5.73; N, 10.89; S, 8.32.
9-(N-piperazinyl)-5-methyl-12(H)-quino[3,4-b][1,4]benzothiazinium chloride (3e). Yield: Procedure (A) 66%, Procedure (B) 74%; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.28–3.32 (m, 4H, Hpiperazinyl), 3.34–3.39 (m, 4H, Hpiperazinyl), 4.11 (s, 3H, NCH3) 6.65–6.69 (d, 4J = 3 Hz, 1H, H8), 6.72–6.77 (d, 3J = 9 Hz, 1H, H11), 6.77–6.82 (d.d, 3J = 9 Hz, 4J = 3 Hz, 1H, H10), 7.75–7.83 (m, 1H, Harom), 7.97–8.04 (m, 2H, Harom), 8.26 (s, 1H, H6), 8.48–8.53 (m, 1H, Harom); Anal. calcd. for C20H21ClN4S: C, 62.41; H, 5.50; N, 14.56; S, 8.33. Found C, 62.32; H, 5.44; N, 14.47; S, 8.28.
3.1.3. Synthesis of 10-Hydroxy-5-methyl-5(H)-quino[3,4-b][1,4]benzothiazine 4
Quinobenzothiazinium chloride (3h) (0.317 g, 1 mmol) was dissolved in 20 mL water (50 °C), the resulting solution was filtered and alkalized while mixing by using a 5% aqueous NaHCO3 solution (10 mL). The obtained solid product was filtered off and air-dried. Finally, the crude product was purified by recrystallization from ethanol.
10-Hydroxy-5-methyl-5(H)-quino[3,4-b][1,4]benzothiazine (4). Yield: 100%; m.p. 107–110 °C; 1H-NMR (CD3ODd-4, 600 MHz) δ (ppm): 3.90 (s, 3H, CH3), 6.42–6.47 (d.d, 3J = 8.4 Hz, 4J = 2.4 Hz, 1H, H9), 6.58 (s, 1H, H6), 6.61–6.67 (m, 1H, Harom), 7.53–7.61 (m, 1H, Harom), 7.66–7.74 (m, 2H, Harom), 7.78–7.86 (m, 1H, Harom), 8.34–8.39 (m, 1H, Harom); EI-MS (70 eV) (m/z): 280 (M+, 100%); Anal. calcd. for C16H12N2OS: C, 68.55; H, 4.31; N, 9.99; S, 11.44. Found: C, 68.53; H, 4.26; N, 9.94; S, 11.42.
3.1.4. Synthesis of 10-Alkoxy-5-methyl-5(H)-quino[3,4-b][1,4]benzothiazine 5
Anhydrous 1,4-dioxane (15 mL) was mixed with quinobenzothiazine (4) (0.28 g, 1 mmol) and sodium hydroxide (0.2 g, 5 mmol) and refluxed with mixing for 2 h. An alkylating agent (alkyl iodides or aminoalkyl chlorides) (1.3 mmol) was added stepwise and the mixture was refluxed for a subsequent 2 h. After cooling down to room temperature, the reaction mixture was poured into 50 mL of water and extracted with 15 mL chloroform. The resulting solution was dried over anhydrous calcium chloride and evaporated under vacuum. The dry residue was purified by chromatography using a silica gel-filled column and chloroform-ethanol (10:1 v/v) as eluent.
10-Methoxy-5-methyl-5(H)-quino[3,4-b][1,4]benzothiazine (5a). Yield: 30%; m.p. 268–270 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.51 (s, 3H, NCH3), 3.68 (s, 3H, OCH3), 6.38–6.44 (m, 2H, Harom), 6.56–6.60 (m, 1H, Harom), 7.07 (s, 1H, H6), 7.21–7.26 (m, 1H, Harom), 7.27–7.30 (m, 1H, Harom), 7.52–7.57 (m, 1H, Harom), 8.18–8.22 (m, 1H, Harom); 13C-NMR (DMSOd-6, 150.9 MHz) δ (ppm): 40.51 (NCH3), 55.46 (OCH3), 103.64 (12b), 111.09 (7a), 111.33(C9 or C11), 112.53 (C9 or C11), 115.89 (C4), 121.29 (6a), 123.96 (C2), 125.02 (C1), 126.01 (C8), 131.98 (3), 133.14 (C6), 140.75 (4a), 146.30 (C11a), 154.13 (C12a), 159.46 (C10); EI-MS (70 eV) (m/z): 294 (M+, 100%); Anal. calcd. for C17H14N2OS: C, 69.36; H, 4.79; N, 9.52; S, 10.89. Found: C, 69.29; H, 4.75; N, 9.48; S, 10.84.
10-Butyloxy-5-methyl-5(H)-quino[3,4-b][1,4]benzothiazine (5b). Yield: 37%; m.p. 280–282 °C; 1H-NMR (CDCl3, 600 MHz) δ (ppm): 2.58 (m, 3H, CH3), 2.70–2.80 (m, 2H, CH2), 3.38–3.50 (m, 2H, CH2), 3.76 (s, 3H, NCH3), 4.03–4.11 (t, J = 4.8 Hz, 2H, OCH2), 6.31–6.45 (m, 2H, Harom), 6.52–6.54 (m, 1H, Harom), 6.54–6.63 (m, 1H, Harom), 6.95–7.03 (m, 1H, Harom), 7.18–7.25 (m, 1H, Harom), 7.40–7.50 (m, 1H, Harom), 8.30–8.45 (m, 1H, Harom); EI-MS (70 eV) (m/z): 336 (M+, 100%); Anal. calcd. for C20H20N2OS: C, 71.40; H, 5.99; N, 8.33; S, 9.53. Found: C, 71.30; H, 5.94; N, 8.28; S, 9.49.
10-(3-(N,N-dimethylamino)propyl)oxy-5-methyl-5(H)-quino[3,4-b][1,4]benzothiazine (5c). Yield: 49%; m.p. 82–83 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 2.10–2.20 (t, 3J = 6 Hz, 2H, NCH2), 2.76 (s, 3H, NCH3), 2.77 (s, 3H, NCH3), 3.11–3.25 (m, 2H, CH2), 3.95–4.05 (t, 3J = 6 Hz, 2H, OCH2), 4.15 (s, 3H, NCH3), 6.65–6.75 (d.d, 3J = 8.4 Hz, 4J = 2.4 Hz, 1H, H9), 6.92–7.00 (d, 3J = 8.4 Hz, 1H, H8), 7.45–7.50 (d, 4J = 2.4 Hz, 1H, H11), 7.75–7.84 (m, 1H, Harom), 7.95–8.12 (m, 2H, Harom), 8.69 (s, 1H, H6), 9.15–9.27 (m, 1H, Harom); EI-MS (70 eV) (m/z): 365 (M+, 100%); Anal. calcd. for C21H23N3OS: C, 69.01; H, 6.34; N, 11.50; S, 8.77. Found: C, 68.96; H, 6.30; N, 11.47; S, 8.75.
10-(2-(N-pyrrolidinyl)ethyl)oxy-5-methyl-5(H)-quino[3,4-b][1,4]benzothiazine (5d). Yield: 46%; m.p. 148–150 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 1.62–1.76 (m, 4H, Hpyrrolidinyl), 2.52–2.57 (m, 4H, Hpyrrolidinyl), 2.73 (t, J = 6 Hz, 2H, NCH2), 3.51 (s, 3H, NCH3), 3.98 (t, J = 6 Hz, 2H, OCH2), 6.37–6.44 (m, 2H, Harom), 6.55–6.59 (m, 1H, Harom), 7.07 (s, 1H, H6), 7.22–7.25 (m, 1H, Harom), 7.25–7.30 (m, 1H, Harom), 7.52–7.57 (m, 1H, Harom), 8.17–8.24 (m, 1H, Harom); EI-MS (70 eV) (m/z): 377 (M+, 73%); Anal. calcd. for: C22H23N3OS: C, 70.00; H, 6.14; N, 11.13; S, 8.49. Found: C, 69.96; H, 6.08; N, 11.09; S, 8.48.
10-(2-(N-piperidinyl)ethyl)oxy-5-methyl-5(H)-quino[3,4-b][1,4]benzothiazine (5e). Yield: 67%; m.p. 70–73 °C; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 1.30–1.40 (m, 2H, Hpiperidinyl), 1.40–1.53 (m, 4H, Hpiperidinyl), 2.35–2.45 (m, 4H, Hpiperidinyl), 2.54–2.61 (t, J = 6 Hz, 2H, NCH2), 3.51 (s, 3H, NCH3), 3.93–4.01 (t, 3J = 6 Hz, 2H, OCH2), 6.35–6.40 (m, 2H, Harom), 6.52–6.59 (m, 1H, Harom), 7.06 (s, 1H, H6), 7.15–7.32 (m, 2H, Harom), 7.49–7.55 (m, 1H, Harom), 8.15–8.22 (m, 1H, Harom); EI-MS (70 eV) (m/z): 392 (M+, 100%); Anal. calcd. for C23H25N3OS: C, 70.56; H, 6.44; N, 10.73; S, 8.19. Found: C, 70.48; H, 6.49; N, 10.70; S, 8.17.
10-(2-(N-morpholinyl)ethyl)oxy-5-methyl-5(H)-quino[3,4-b][1,4]benzothiazine (5f). Yield: 15%; m.p. 196–198 °C; 1H-NMR (DMSOd6, 600 MHz) δ (ppm): 2.38–2.45 (m, 4H, Hmorpholinyl), 2.57–2.65 (t, J = 5.4 Hz, 2H, NCH2), 3.51 (s, 2H, NCH3), 3.53–3.62 (m, 4H, Hmorpholinyl), 3.92–4.05 (t, J = 5.4 Hz, 2H, OCH2), 6.38–6.44 (m, 2H, Harom), 6.55–6.59 (m, 1H, Harom), 7.07 (s, 1H, H6), 7.20–7.32 (m, 2H, Harom), 7.48–7.57 (m, 1H, Harom), 8.18–8.24 (m, 1H, Harom). EI-MS (70 eV) (m/z): 394 (M+, 100%); Anal. calcd. for C22H23N3O2S: C, 67.15; H, 5.89; N, 10.68; S, 8.15. Found: C, 67.10; H, 5.83; N, 10.62; S 8.12.
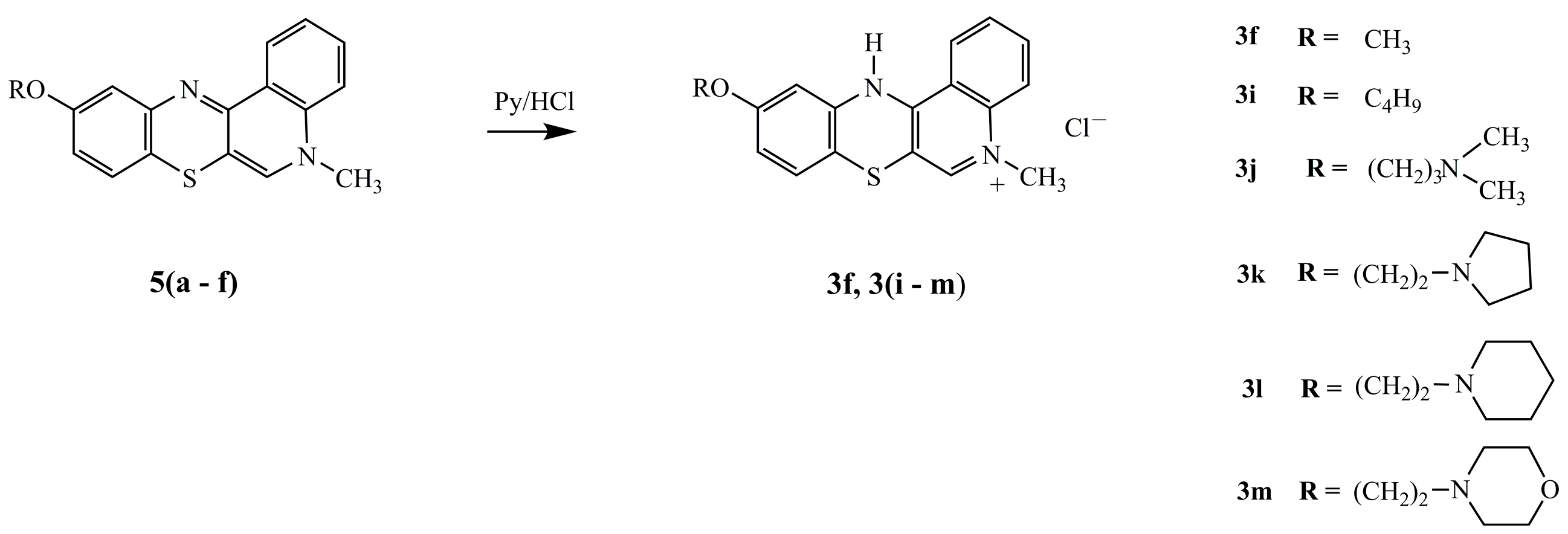
3.1.5. Synthesis of 12(H)-quino[3,4-b][1,4]benzothiazinium Chloride 3f, 3i–m
Quinobenzothiazine (5) (1 mmol) was dissolved in 15 mL anhydrous ethanol. Pyridine hydrochloride (1 mmol) was added and the reaction mix was refluxed for 2 h. Solvents were evaporated under vacuum and the dried remaining residue was purified using a chromatography column filled with aluminum oxide and chloroform:ethanol (10:1 v/v) as eluent.
10-Methoxy-5-methyl-12(H)-quino[3,4-b][1,4]benzothiazinium chloride (3f).Yield: 87%; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.75 (s, 3H, NCH3), 4.15 (s, 3H, OCH3), 6.68–6.72 (m, 1H, Harom), 6.98–7.02 (m, 1H, Harom), 7.24–7.26 (d, 4J = 3 Hz, H11), 7.83–7.88 (m, 1H, Harom), 8.01–8.12 (m, 2H, Harom), 8.68 (s, 1H, H6), 8.92–8.98 (m, 1H, Harom), 10.97 (s, 1H, NH). Anal. calcd. for C17H15ClN2OS: C, 61.72; H, 4.57; N, 8.47; S, 9.69. Found: C, 61.65; H, 4.51; N, 8.42; S, 9.66.
10-Butyloxy-5-methyl-12(H)-quino[3,4-b][1,4]benzothiazinium chloride (3i). Yield 82%; 1H-NMR (DMSOd-6, 600 MHZ) δ (ppm): 3.15–3.25 (m, 3H, CH3), 3.71–3.85 (m, 2H, CH2), 3.95–4.02 (m, 2H, CH2), 4.15 (s, 3H, NCH3), 4.34–4.43 (t, J = 4.2 Hz, 2H, OCH2), 6.75–6.80 (d.d, 3J = 8.4 Hz, 4J = 2.4 Hz, 1H, H9), 7.03–7.08 (d, 3J = 8.4 Hz, 1H, H8), 7.25–7.31 (d, 4J =2.4 Hz, 1H, H11), 7.82–7.89 (m, 1H, Harom), 8.04–8.12 (m, 2H, Harom), 8.69 (s, 1H, H6), 8.91–8.97 (m, 1H, Harom), 11.11 (s, 1H, NH). Anal. calcd. for C20H21ClN2OS: C, 64.42; H, 5.68; N, 7.51; S, 8.60. Found: C, 64.38; H, 5.63; N, 7.75; S 8.57.
10-(3-(N,N-Dimethylamino)propyl)oxy-5-methyl-12(H)-quino[3,4-b][1,4]benzothiazinium chloride (3j). Yield: 80%; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 2.16 (t, J = 6 Hz, 2H, NCH2), 2.77 (s, 3H, NCH3), 2.78 (s, 3H, NCH3), 3.19 (m, 2H, CH2), 4.04 (t, J = 6 Hz, 2H, OCH2), 4.15 (s, 3H, NCH3), 6.67–6.73 (d.d, 3J = 8.4 Hz, 4J = 2.4 Hz, 1H, H9), 6.98–7.02 (d, 3J = 8.4 Hz, 1H, H8), 7.48–7.53 (d, 4J = 2.4 Hz, 1H, H11), 7.78–7.85 (m, 1H, Harom), 8.02–8.11 (m, 2H, Harom), 8.69 (s, 1H, H6), 9.20–9.25 (m, 1H, Harom), 10.73–10.80 (m, 1H, Harom), 11.39 (s, 1H, NH); 13C-NMR (DMSOd-6, 150.9 MHz) δ (ppm): 24.16 (NCH2), 42.49 (N(CH3)2), 43.11 (NCH3), 54.30 (CH2), 65.72 (OCH2), 106.67 (C6a), 106.96 (C11), 107.40 (7a), 113.22 (C9), 116.17 (11a), 119.16 (C4), 124.88 (C1), 127.92 (C8), 128.23 (C12a), 134.85 (C2), 138.05 (C3), 139.10 (C4a), 143.69 (C6), 151.87 C12a), 158.92 (C10); Anal. calcd. for C21H24ClN3OS: C, 62.75; H, 6.02; N, 10.45; S, 7.98. Found: C, 62.71; H, 5.96; N, 10.40; S, 7.94.
10-(2-(N-Pyrrolidinyl)ethyl)oxy-5-methyl-12(H)-quino[3,4-b][1,4]benzothiazinium chloride (3k). Yield: 83%; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 1.80–1.90 (m, 2H, Hpyrrolidinyl), 1.90–2.05 (m, 2H, Hpyrrolidinyl), 3.02–3.12 (m, 2H, NCH2), 3.51–3.62 (m, 4H, Hpyrrolidinyl), 4.11 (s, 3H, NCH3), 4.30–4.35 (t, J = 4.8 Hz, 2H, OCH2), 6.80–6.85 (m, 2H, Harom), 7.50–7.56 (m, 1H, Harom), 7.78–7.84 (m, 1H, Harom), 8.02–8.07 (m, 1H, Harom), 8.61 (s, 1H, H6), 8.95–9.00 (m, 1H, Harom), 10.95–11.04 (m, 1H, Harom), 11.13 (s, 1H, NH); Anal. calcd. for C22H24ClN3OS: C, 63.83; H, 5.84; N, 10.15; S, 7.74. Found: C, 63.86; H, 5.78; N, 10.11; S, 7.70.
10-(2-(N-Piperidinyl)ethyl)oxy-5-methyl-12(H)-quino[3,4-b][1,4]benzothiazinium chloride (3l). Yield: 86%; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 1.32–1.45 (m, 2H, Hpiperydyl), 1.70–1.90 (m, 4H, Hpiperydyl), 2.95–3.10 (t, J = 4.8 Hz, 2H, NCH2), 3.45–3.50 (m, 4H, Hpiperydyl), 4.15 (s, 3H, NCH3), 4.38–4.43 (t, J = 4.8 Hz, 2H, OCH2), 6.73–6.78 (d.d, 3J = 9 Hz, 4J = 3 Hz, 1H, H9), 7.01–7.05 (d, 3J = 9 Hz, 1H, H8), 7.32–7.34 (d, 3J = 3 Hz, 1H, H11), 7.81–7.88 (m, 1H, Harom), 8.01–8.10 (m, 2H, Harom), 8.69 (s, 1H, H6), 8.98–9.04 (m, 1H, Harom), 10.46 (s, 1H, NH). Anal. calcd. for C23H26ClN3OS: C, 64.55; H, 6.12; N, 9.82; S, 7.49. Found: C, 64.50; H, 6.06; N, 9.78; S, 7.45.
10-(2-(N-morpholinyl)ethyl)oxy-5-methyl-12(H)-quino[3,4-b][1,4]benzothiazinium chloride (3m). Yield: 100%; 1H-NMR (DMSOd-6, 600 MHz) δ (ppm): 3.12–3.33 (m, 4H, Hmorpholinyl), 3.85–4.02 (m, 6H, NCH2, 4Hmorpholinyl), 4.16 (s, 3H, NCH3), 4.38–4.45 (t, J = 4.2 Hz, OCH2), 6.75–6.78 (d.d, 3J = 9 Hz, 4J = 2.4 Hz, H9), 7.02–7.06 (d, 3J = 9 Hz, 1H, H8), 7.48–7.53 (d, 4J = 2.4 Hz, 1H, H11), 7.78–7.68 (m, 1H, Harom), 8.03–8.12 (m, 2H, Harom), 8.71 (s, 1H, H6), 9.16–9.20 (m, 1H, Harom), 11.38 (s, 1H, NH). Anal. calcd. for C22H24ClN3OS: C, 61.46; H, 5.63; N, 9.77; S, 7.46. Found: C, 61.42; H, 5.59; N, 7.73; S, 7.44.