Bauhinia championii Flavone Attenuates Hypoxia-Reoxygenation Induced Apoptosis in H9c2 Cardiomyocytes by Improving Mitochondrial Dysfunction
Abstract
:1. Introduction
2. Results
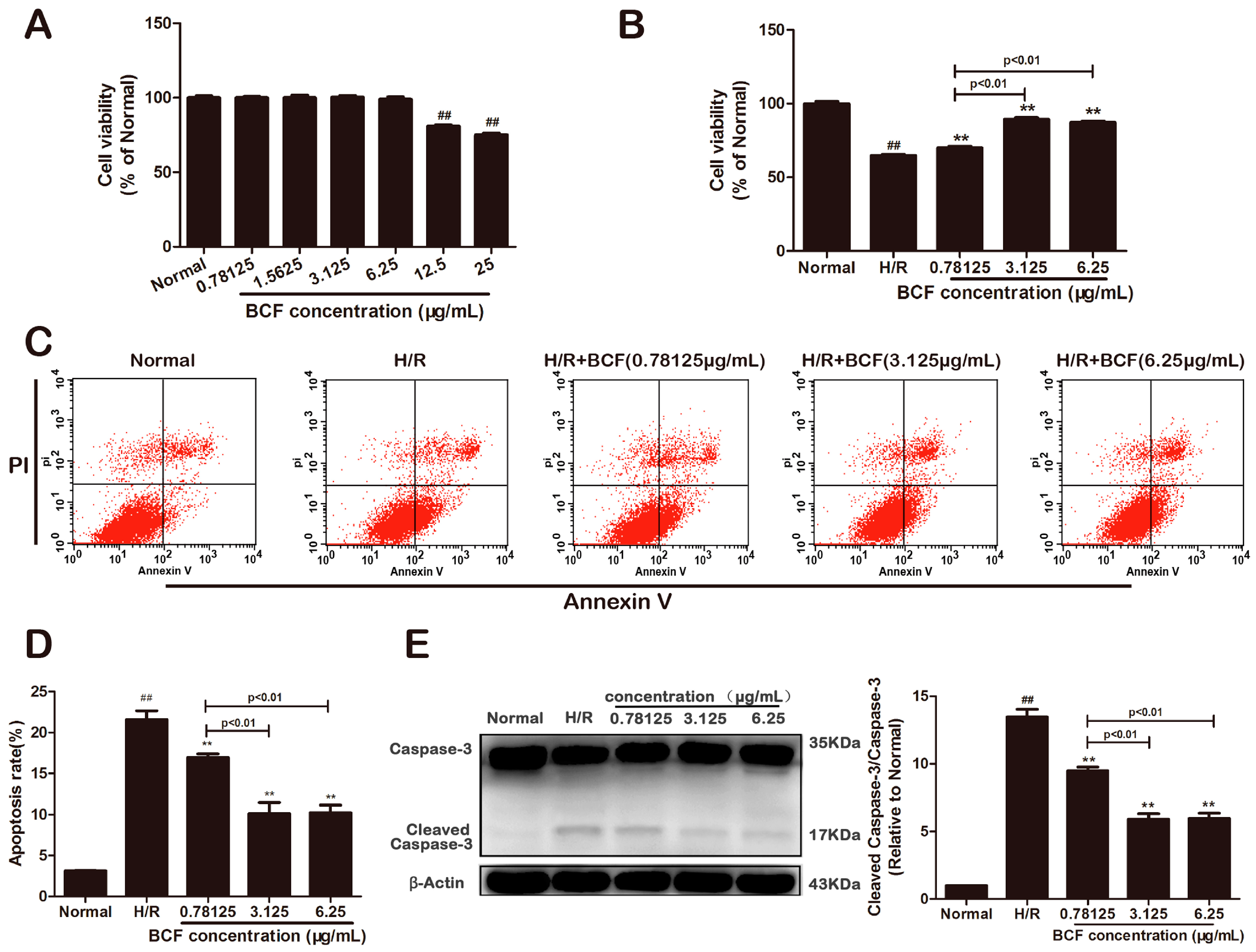
2.1. BCF Mitigated H/R Induced Apoptosis
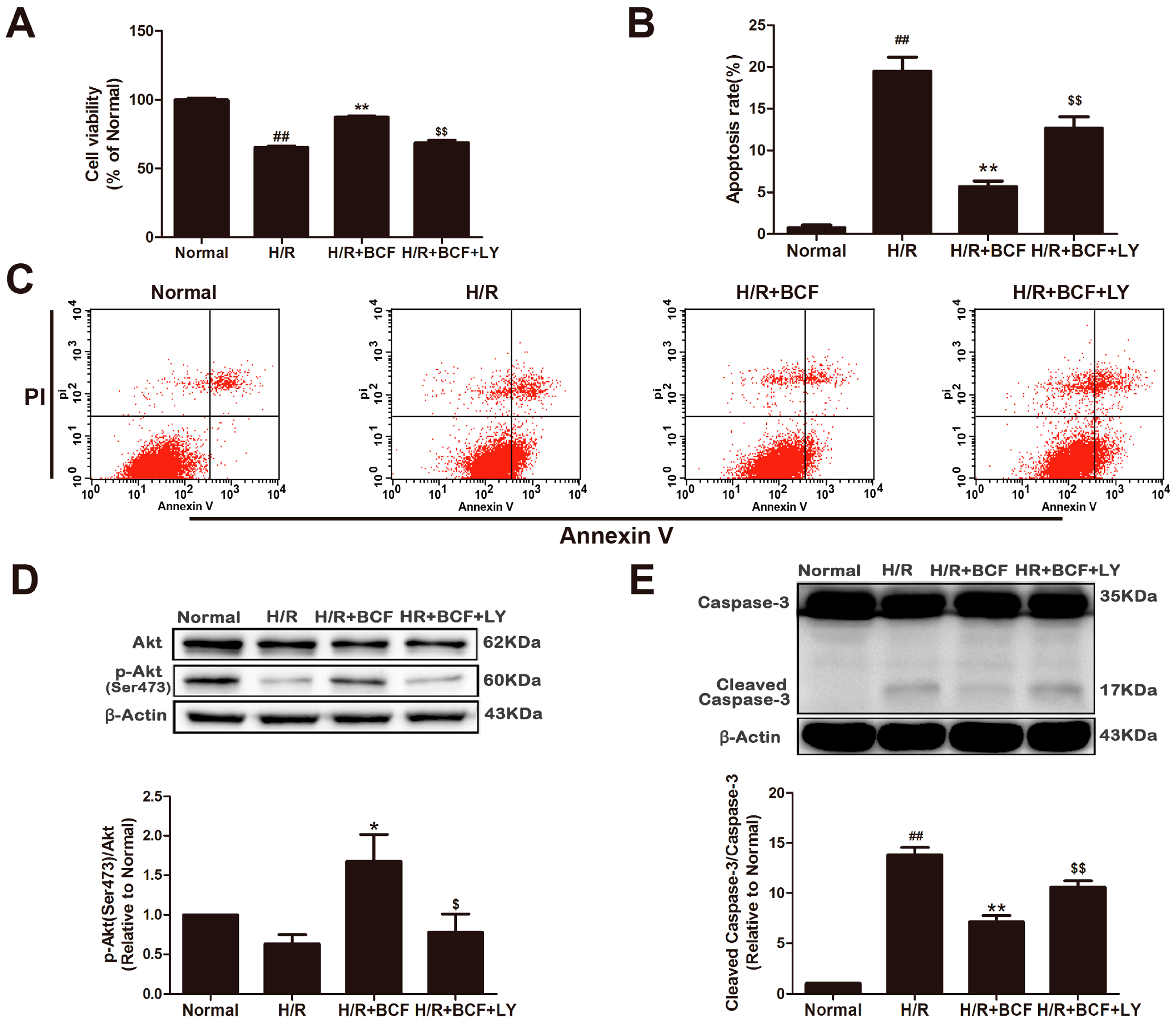
2.2. BCF Pretreatment Activated PI3K/Akt Signaling Pathway
2.3. Inhibition of PI3K Attenuated BCF-Induced Cardioprotection
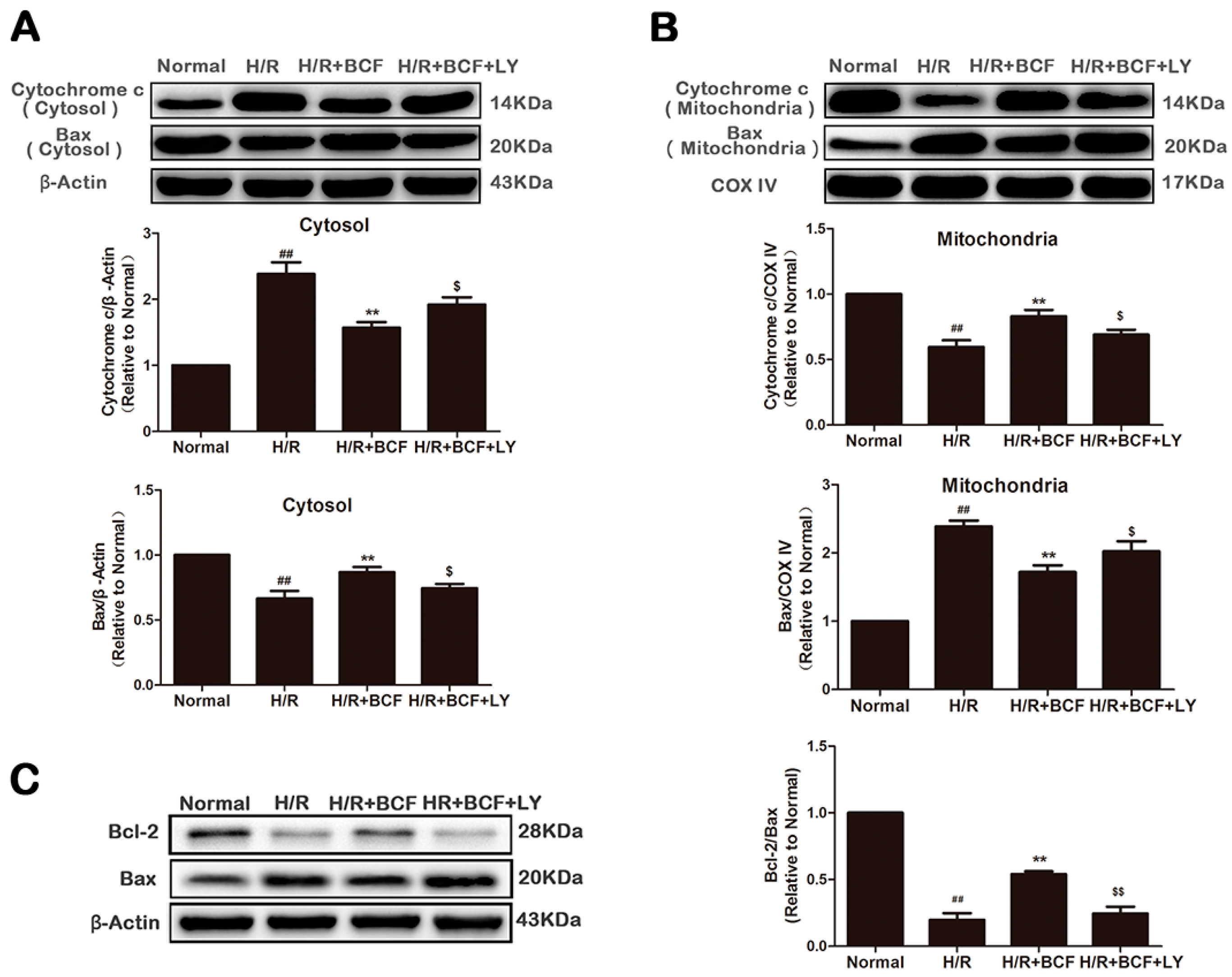
2.4. BCF Inhibited H/R-Induced Mitochondrial Dysfunction via PI3K/Akt Signaling Pathway
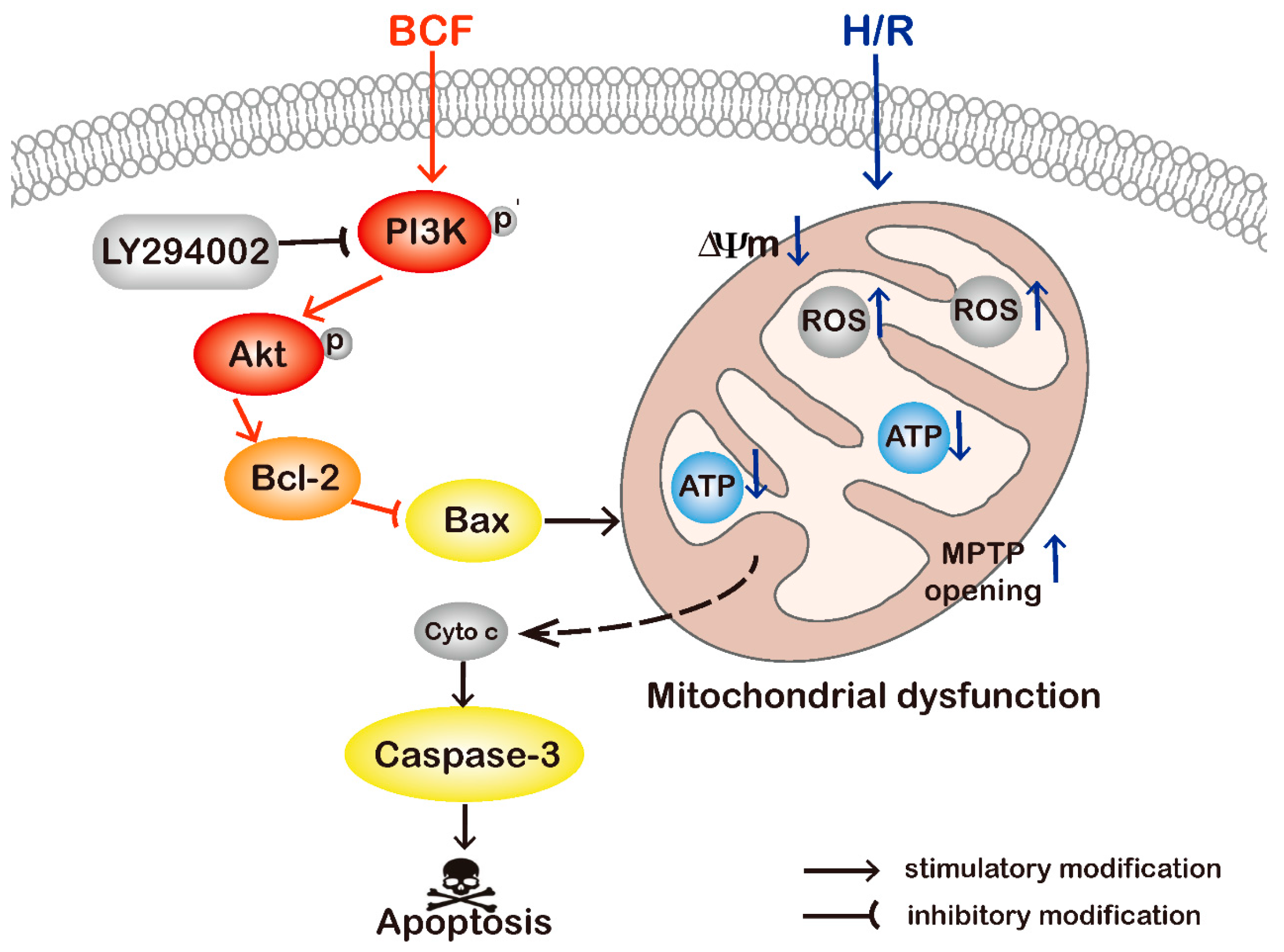
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Cell Culture and Hypoxia-Reoxygenation Model (H/R)
4.3. Experimental Protocols
4.4. Cell Viability Analysis
4.5. Flow Cytometric Detection of Apoptosis
4.6. Measurement of ROS-Generating Mitochondria
4.7. Determination of Mitochondrial Transmembrane Potential (∆Ψm) and ATP Production
4.8. Determination of Changes in the MPTP
4.9. Western Blotting Analysis
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Head, S.J.; Kappetein, A.P. Coronary artery disease: A dam in the river for ranolazine. Lancet 2016, 387, 100–102. [Google Scholar] [CrossRef]
- Bao, M.H.; Luo, H.Q.; Xiang, J.; Tang, L.; Dong, L.P.; Li, G.Y.; Zeng, J.; Li, J.M. Meta-Analysis for the Association between Polymorphisms in Interleukin-17A and Risk of Coronary Artery Disease. Int. J. Environ. Res. Public Health 2016, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lin, Y.; Tang, X.; Zhang, P.; Liu, B.; Liu, Y.; Miao, F. Helix B surface peptide protects cardiomyocytes against hypoxia/reoxygenation-induced apoptosis through mitochondrial pathways. J. Cardiovasc. Pharmacol. 2016, 67, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, P.M.; Crovella, S.; Celsi, F. Mevalonate pathway blockade, mitochondrial dysfunction and autophagy: A possible link. Int. J. Mol. Sci. 2015, 16, 16067–16084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinidis, K.; Whelan, R.S.; Kitsis, R.N. Mechanisms of cell death in heart disease. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Sun, Z.; Tong, G.; Yi, W.; Ma, L.; Zhao, B.; Cheng, L.; Zhang, J.; Cao, F.; Yi, D. Alpha-Lipoic acid reduces infarct size and preserves cardiac function in rat myocardial ischemia/reperfusion injury through activation of PI3K/Akt/Nrf2 pathway. PLoS ONE 2013, 8, e58371. [Google Scholar]
- Wang, M.; Sun, G.B.; Zhang, J.Y.; Luo, Y.; Yu, Y.L.; Xu, X.D.; Meng, X.B.; Zhang, M.D.; Lin, W.B.; Sun, X.B. Elatoside C protects the heart from ischaemia/reperfusion injury through the modulation of oxidative stress and intracellular Ca2+ homeostasis. Int. J. Cardiol. 2015, 185, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, Q.; Jiang, L.; Guo, C.; Li, Y.; Yu, Y.; Li, Y.; Duan, J.; Sun, Z. DNA hypermethylation of CREB3L1 and Bcl-2 Associated with the mitochondrial-mediated apoptosis via PI3K/Akt pathway in human BEAS-2B cells exposure to silica nanoparticles. PLoS ONE 2016, 11, e0158475. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Q.; Chang, G.L.; Wang, Y.; Zhang, D.Y.; Cao, L.; Liu, J. Geniposide prevents hypoxia/reoxygenation-induced apoptosis in H9c2 Cells: Improvement of mitochondrial dysfunction and activation of GLP-1R and the PI3K/AKT signaling pathway. Cell. Physiol. Biochem. 2016, 39, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chu, K.; Li, H.; Zhang, Y.; Zheng, H.; Chen, R.; Chen, L. Ionic liquid-based microwave-assisted extraction of flavonoids from Bauhinia championii (Benth.) Benth. Molecules 2012, 17, 14323–14335. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chu, K.; Li, H.; Zhang, Y.; Huang, M.; Zheng, H.; Sha, M.; Zhang, X.; Chen, L. Bauhinia championii extraction treatment of collagen-induced arthritis via downregulation of the expression of TLR4, MyD88 and NF-kappaB. Am. J. Chin. Med. 2013, 41, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Xuan, F.; Qin, F.; Huang, R. The antioxidant, anti-inflammatory and anti-apoptotic activities of the Bauhinia championii flavone are connected with protection against myocardial ischemia/reperfusion injury. Cell. Physiol.Biochem. 2016, 38, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Xuan, F.; Qin, F.; Huang, R. Bauhinia championii flavone inhibits apoptosis and autophagy via the PI3K/Akt pathway in myocardial ischemia/reperfusion injury in rats. Drug Des. Dev. Ther. 2015, 9, 5933–5945. [Google Scholar]
- Wei, K.; Liu, L.; Xie, F.; Hao, X.; Luo, J.; Min, S. Nerve growth factor protects the ischemic heart via attenuation of the endoplasmic reticulum stress induced apoptosis by activation of phosphatidylinositol 3-kinase. Int. J. Med. Sci. 2015, 12, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; Liu, Y.; Wu, Y.; Li, H.; Zhao, C.; Li, H.; Meng, Q.; Li, W. Effect of hematoporphyrin monomethyl ether-sonodynamic therapy (HMME-SDT) on hypertrophic scarring. PLoS ONE 2014, 9, e86003. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, Q.; Zhang, J.; Cao, B.; Zhao, P.; Qin, X. In vitro activation of mitochondria-caspase signaling pathway in sonodynamic therapy-induced apoptosis in sarcoma 180 cells. Ultrasonics 2010, 50, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef] [PubMed]
- Du, J.K.; Cong, B.H.; Yu, Q.; Wang, H.; Wang, L.; Wang, C.N.; Tang, X.L.; Lu, J.Q.; Zhu, X.Y.; Ni, X. Upregulation of microRNA-22 contributes to myocardial ischemia-reperfusion injury by interfering with the mitochondrial function. Free Radic. Biol. Med. 2016, 96, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Chipuk, J.E.; Moldoveanu, T.; Llambi, F.; Parsons, M.J.; Green, D.R. The BCL-2 family reunion. Mol. Cell 2010, 37, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Goping, I.S.; Gross, A.; Lavoie, J.N.; Nguyen, M.; Jemmerson, R.; Roth, K.; Korsmeyer, S.J.; Shore, G.C. Regulated targeting of BAX to mitochondria. J. Cell Biol. 1998, 143, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Farombi, E.O. Atrazine induces apoptosis of SH-SY5Y human neuroblastoma cells via the regulation of Bax/Bcl-2 ratio and caspase-3-dependent pathway. Pestic. Biochem. Physiol. 2015, 118, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.M.; Aljada, A.; El-Abadelah, M.M.; Sabri, S.S.; Zahra, J.A.; Nasr, A.; Aziz, M.A. The pyridone-annelated isoindigo (5’-Cl) induces apoptosis, dysregulation of mitochondria and formation of ROS in leukemic HL-60 cells. Cell. Physiol. Biochem. 2015, 35, 1958–1974. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Ye, T.; Wang, M.; Xia, Y.; Wang, N.; Song, X.; Wang, F.; Liu, L.; Zhu, Y.; Yang, F.; et al. A novel cinnamide YLT26 induces breast cancer cells apoptosis via ROS-mitochondrial apoptotic pathway in vitro and inhibits lung metastasis in vivo. Cell. Physiol. Biochem. 2014, 34, 1863–1876. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, G.; Meng, X.; Wang, H.; Wang, M.; Qin, M.; Ma, B.; Luo, Y.; Yu, Y.; Chen, R.; et al. Ginsenoside RK3 prevents hypoxia-reoxygenation induced apoptosis in H9c2 cardiomyocytes via AKT and MAPK pathway. Evid. Based Complement. Alternat. Med. 2013, 2013, 690190. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Li, G.H.; Peng, L.J.; Qu, S.L.; Zhang, Y.; Peng, J.; Luo, X.Y.; Hu, H.J.; Ren, Z.; Liu, Y.; et al. PI3K/Akt/FoxO3a signaling mediates cardioprotection of FGF-2 against hydrogen peroxide-induced apoptosis in H9c2 cells. Mol. Cell. Biochem. 2016, 414, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, P.; Sun, G.; Zhang, C.; Wang, M.; Sun, Y.; Zhou, Y.; Sun, X.; Jian, J. Bauhinia championii Flavone Attenuates Hypoxia-Reoxygenation Induced Apoptosis in H9c2 Cardiomyocytes by Improving Mitochondrial Dysfunction. Molecules 2016, 21, 1469. https://doi.org/10.3390/molecules21111469
Liao P, Sun G, Zhang C, Wang M, Sun Y, Zhou Y, Sun X, Jian J. Bauhinia championii Flavone Attenuates Hypoxia-Reoxygenation Induced Apoptosis in H9c2 Cardiomyocytes by Improving Mitochondrial Dysfunction. Molecules. 2016; 21(11):1469. https://doi.org/10.3390/molecules21111469
Chicago/Turabian StyleLiao, Ping, Guibo Sun, Chan Zhang, Min Wang, Yao Sun, Yuehan Zhou, Xiaobo Sun, and Jie Jian. 2016. "Bauhinia championii Flavone Attenuates Hypoxia-Reoxygenation Induced Apoptosis in H9c2 Cardiomyocytes by Improving Mitochondrial Dysfunction" Molecules 21, no. 11: 1469. https://doi.org/10.3390/molecules21111469
APA StyleLiao, P., Sun, G., Zhang, C., Wang, M., Sun, Y., Zhou, Y., Sun, X., & Jian, J. (2016). Bauhinia championii Flavone Attenuates Hypoxia-Reoxygenation Induced Apoptosis in H9c2 Cardiomyocytes by Improving Mitochondrial Dysfunction. Molecules, 21(11), 1469. https://doi.org/10.3390/molecules21111469




