Are Vicilins Another Major Class of Legume Lectins?
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lectin Activity in Different Lupinus albus Protein Fractions
2.1.1. Presence of Lectin Activities in the Albumin Fraction from Lupinus albus Seeds
| Sugar (at 0.1 M Initial Concentration) | Sugar Minimal Inhibitory Concentration (m.i.c.) (M) | Maximal Protein Concentration Inhibiting 4 H.U.* (μg/mL) |
|---|---|---|
| d-Glucose | 0.1 | 49.5 |
| d-Glucosamine | 0.1 | 49.5 |
| N-Acetyl-d-glucosamine | 0.1 | 49.5 |
| d-Galactose | 1.7 × 10−6 | 49.5 |
| d-Galactosamine | UD** | - |
| Sialic acid | 3.7 × 10−3 | 49.5 |
| Lactose | UD | - |
| d-Mannose | 0.1 | 49.5 |
| Raffinose | 11.1 × 10−3 | 49.5 |
| l-Fucose | 11.1 × 10−3 | 49.5 |
| Melezitose | 3.7 × 10−3 | 49.5 |
| α-Methyl-d-glucopyranoside | UD | 49.5 |
| Sucrose | 3.3 × 10−2 | 49.5 |
2.1.2. γ-Conglutin from Lupinus albus Seeds
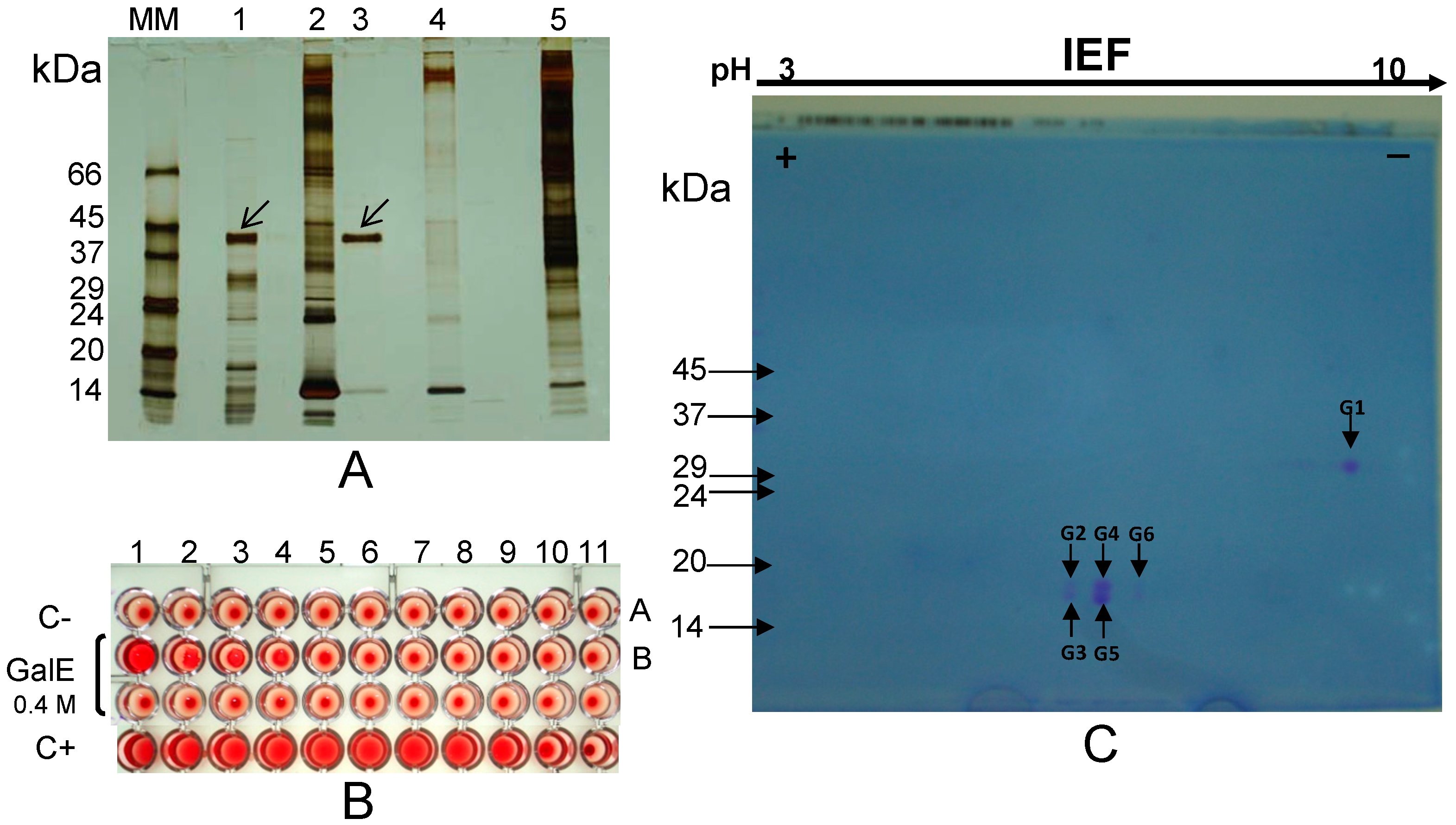

2.1.3. β-Conglutin Fraction from 8 DAG Lupinus albus Cotyledons (Blad-Containing Oligomer)


2.2. Are Vicilins another Major Class of Legume Lectins?
2.2.1. The Lectin Nature of Lupinus albus Vicilin (β-Conglutin) Subunits

2.2.2. The Lectin Nature of Vicia faba Vicilin Subunits
2.2.3. The Lectin Nature of Lathyrus sativus Vicilin (β-Lathyrin) Subunits

2.3. The Lectin Nature of Legume Vicilins
| Legume Species (Seeds) | Immunodetected Polypeptides Specifically Bound to Erythrocyte Membranes (kDa) | Immunodetected Polypeptides from Mannose Eluates (kDa) |
|---|---|---|
| Lupinus albus | 56 ± 2 | NT* |
| 51 ± 2 | ||
| 46 ± 2 | ||
| 43 ± 2 | ||
| 40 ± 2 | ||
| 36 ± 2 | ||
| 31 ± 2 | ||
| Vicia faba | 64 ± 2 | NT |
| 43 ± 2 | ||
| 41 ± 2 | ||
| Lathyrus sativus | 73 ± 2 | 73 ± 2 |
| 71 ± 2 | 71 ± 2 | |
| 61 ± 2 | 61 ± 2 | |
| 48 ± 2 | 57 ± 2 | |
| 40 ± 2 | 48 ± 2 | |
| 28 ± 2 | ||
| 13 ± 2 |
3. Experimental Section
3.1. Plant Material and Growth Conditions
3.2. Isolation of Total Albumins and Total Globulins Based on Solubility Criteria
3.3. Purification of Individual Globulins
3.4. Purification of the Native Oligomer Containing Blad
3.5. Haemagglutination Assays
3.6. Affinity Binding of Lectins to Isolated Erythrocyte Membranes
3.7. Production of Polyclonal Antibodies
3.8. Electrophoresis and Western Blotting
3.9. Immunoblotting
3.10. Polypeptide Sequencing
3.11. General Assays
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Dannielson, C.E. Seed globulin of the Gramineae and Leguminoseae. Biochem. J. 1949, 44, 387–400. [Google Scholar]
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945–956. [Google Scholar] [CrossRef]
- Astwood, J.D.; Silvanovich, A.; Bannon, G.A. Vicilins: A case study in allergen pedigrees. J. Allergy Clin. Immun. 2002, 110, 26–27. [Google Scholar] [CrossRef]
- Macedo, M.L.R.; Fernandes, K.V.S.; Sales, M.P.; Xavier-Filho, J. Purification and properties of storage proteins (vicilins) from cowpea (Vigna unguiculata) seeds which are susceptible or resistant to the bruchid beetle Callosobruchus maculatus. Braz. J. Med. Biol. Res. 1995, 28, 183–190. [Google Scholar]
- Cuadrado, C.; Guilhamón, E.; Goyoga, C.; Pedrosa, M.M.; Altares, P.; Burbano, C.; Muzquiz, M.; Romero, C. Modification of seed storage proteins during germination and seedling growth of faba bean cotyledons. In Recent Advances of Research in Antinutritional Factors in Legume Seeds and Oilseeds; Muzquiz, M., Hill, G.D., Cuadrado, C., Pedrosa, M.M., Burbano, C., Eds.; EAPP Publication: Toledo, Spain, 2004; Volume 110, pp. 307–315. [Google Scholar]
- Radauer, C.; Breiteneder, H. Evolutionary biology of plant food allergens. J. Allergy Clin. Immun. 2007, 120, 518–525. [Google Scholar] [CrossRef]
- Gibbs, P.E.; Strongin, K.B.; McPherson, A. Evolution of legume seed storage proteins—A domain common to legumins and vicilins is duplicated in vicilins. Mol. Biol. Evol. 1989, 6, 614–623. [Google Scholar]
- Ferreira, R.; Freitas, R.; Monteiro, S. Targeting carbohydrates: A novel paradigm for fungal control. Eur. J. Plant Pathol. 2012, 133, 117–140. [Google Scholar] [CrossRef]
- Van Damme, E.J.M.; Peumans, W.J.; Barre, A.; Rougé, P. Plant lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 1998, 17, 575–692. [Google Scholar]
- Rüdiger, H.; Gabius, H.-J. Review. Plant lectins: Occurrence, biochemistry, functions and applications. Glycoconjugate J. 2001, 18, 589–613. [Google Scholar] [CrossRef]
- Rüdiger, H.; Gabius, H.-J. Plant lectins. In The Sugar Code. Fundamentals of Glycosciences, 1st ed.; Gabius, H.-J., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 301–315. [Google Scholar]
- Chrispeels, M.J.; Raikhel, N.V. Lectins, lectins genes and their role in plant defense. Plant Cell 1991, 3, 1–9. [Google Scholar] [CrossRef]
- Peumans, W.J.; van Damme, E.J.M. Lectins as plant defense proteins. Plant Physiol. 1995, 109, 347–352. [Google Scholar] [CrossRef]
- Down, R.E.; Gatehouse, A.M.R.; Hamilton, W.D.O.; Gattehouse, J.A. Snowdrop lectin inhibits development and decreases fecundity of the glasshouse potato aphid (Aulacorthum solani) when ad ministered in vitro and via transgenic plants both in laboratory and glasshouse trials. Insect Physiol. 1996, 42, 1035–1045. [Google Scholar] [CrossRef]
- Gatehouse, A.M.R.; Davidson, G.M.; Newell, C.A.; Merryweather, A.; Hamilton, W.D.O.; Burgess, E.P.J.; Gilbert, R.J.C.; Gathehouse, J.A. Trangenic potato plants with enhanced resistence to the tomato moth Lacanobia oleraceae: Growth room trials. Mol. Breed. 1997, 3, 49–63. [Google Scholar] [CrossRef]
- Tinjuangiun, P.; Loe, N.T.; Gatehouse, A.M.R.; Gatehouse, J.A.; Christou, P. Enhanced insect resistence in Thai rice varieties generated by particle bombardment. Mol. Breed. 2000, 6, 391–399. [Google Scholar] [CrossRef]
- Ng, T.B. Review. Antifungal proteins and peptides of leguminous and non-leguminous origins. Peptides 2004, 25, 1215–1222. [Google Scholar] [CrossRef]
- Gatehouse, A.M.R.; Powell, K.S.; van Damme, E.J.M.; Peumans, W.J.; Gatehouse, J.A. Insecticidal properties of plant lectins: Their potential in plant protection. In Lectins: Biomedical Perspectives; Pustzai, A., Bardocz, S., Eds.; Taylor and Francis: London, UK, 1995; pp. 35–58. [Google Scholar]
- Schuler, T.H.; Poppy, G.M.; Kerry, B.R.; Denholm, I. Insect-resistant transgenic plants. Trends Biotechnol. 1998, 16, 168–174. [Google Scholar] [CrossRef]
- Brewing, N.J.; Kardailsky, I.V. Legume lectins and nodulation by Rhizobium. Trends Plant Sci. 1997, 2, 92–98. [Google Scholar] [CrossRef]
- Carlini, C.R.; Grossi-de-Sá, M.F. Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides. Toxicon 2002, 40, 1515–1539. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Monteiro, S.; Loureiro, V.; Teixeira, A. Protein Extracted from Plants of the Genus Lupinus or Produced in Recombinant Form, Nucleotide Sequence Encoding It and Its Use in Animal Nutrition, as a Plant Growth Promoter and in the Fight against Pathogenic Fungi. European Patent No. 1907550, 17 October 2012. [Google Scholar]
- Sharon, N.; Lis, H. Legume lectins—A large family of homologous proteins. FASEB J. 1990, 4, 3198–3208. [Google Scholar]
- Vijayan, M.; Chandra, N. Lectins. Curr. Opin. Struct. Biol. 1999, 9, 707–714. [Google Scholar] [CrossRef]
- Gomes, V.M.; Okorokov, L.A.; Rose, T.L.; Fernandes, K.V.S.; Xavier-Filho, J. Legume vicilins (7S storage globulins) inhibit yeast growth and glucose stimulated acidification of the medium by yeast cells. Biochim. Biophys. Acta 1998, 1379, 207–216. [Google Scholar] [CrossRef]
- Firmino, F.; Fernandes, K.V.S.; Sales, M.P.; Gomes, V.M.; Miranda, M.R.A.; Domingues, S.J.S.; Xavier-Filho, J. Cowpea (Vigna unguiculata) vicilins associate with putative chitinous structures in midgut and feces of the bruchid beetles Callosobruchus maculatus and Zabrotes subfasciatus. Braz. J. Med. Biol. Res. 1996, 29, 749–756. [Google Scholar]
- Uchôa, A.F.; DaMatta, R.A.; Retamal, C.A.; Albuquerque-Cunha, J.M.; Souza, S.M.; Samuels, R.I.; Silva, C.P.; Xavier-Filho, J. Presence of the storage seed protein vicilin in internal organs of larval Callosobruchus maculatus (Coleoptera: Bruchidae). J. Insect Physiol. 2006, 52, 169–178. [Google Scholar] [CrossRef]
- Souza, S.M.; Uchôa, A.F.; Silva, J.R.; Samuels, R.I.; Oliveira, A.E.A.; Oliveira, E.M.; Linharesf, R.T.; Alexandre, D.; Silva, C.P. The fate of vicilins, 7S storage globulins, in larvae and adult Callosobruchus maculatus (Coleoptera: Chrysomelidae: Bruchinae). J. Insect Physiol. 2010, 56, 1130–1138. [Google Scholar] [CrossRef]
- Ribeiro, A.; Catarino, S.; Ferreira, R.B.F. Multiple lectin detection by cell membrane affinity binding. Carbohyd. Res. 2012, 352, 206–210. [Google Scholar] [CrossRef]
- Ramos, P.C.R.; Ferreira, R.M.S.B.; Franco, E.; Teixeira, A.R.N. Accumulation of a lectin-like breakdown product of β-conglutin catabolism in cotyledons of germinating Lupinus albus L. seeds. Planta 1997, 203, 26–34. [Google Scholar]
- Seabra, M.; Carvalho, S.; Freire, J.; Ferreira, R.; Mourato, M.; Cunha, L.; Cabral, F.; Teixeira, A.; Aumaitre, A. Lupinus luteus, Vicia sativa and Lathyrus cicera as protein sources for piglets: Ileal and total tract apparent digestibility of amino acids antigenic effects. Anim. Feed Sci. Technol. 2001, 89, 1–16. [Google Scholar] [CrossRef]
- Salgado, P.; Freire, J.P.B.; Mourato, M.; Cabral, F.; Toullec, R.; Lallès, J.P. Comparative effects of different legume protein sources in weaned piglets: Nutrient digestibility, intestinal morphology and digestive enzymes. Livest. Prod. Sci. 2002, 74, 191–202. [Google Scholar] [CrossRef]
- Salgado, P.; Freire, J.P.B.; Ferreira, R.B.; Teixeira, A.; Bento, O.B.; Abreu, M.C.; Toullec, R.; Lallès, J.-P. Immunodetection of legume proteins resistant to small intestinal digestion in weaned piglets. J. Sci. Food Agric. 2003, 83, 1571–1580. [Google Scholar] [CrossRef]
- Rosa, M.J.S.; Ferreira, R.B.; Teixeira, A.R. Storage proteins from Lathyrus sativus seeds. J. Agric. Food Chem. 2000, 48, 5432–5439. [Google Scholar] [CrossRef]
- Duranti, M.; Faoro, F.; Harris, N. The unusual extracellular localization of conglutin γ in germinating Lupinus albus seeds rules out its role as a storage protein. J. Plant Physiol. 1994, 143, 711–716. [Google Scholar] [CrossRef]
- Duranti, M.; Gius, C.; Scarafoni, A. Lectin-like activity of lupin seed conglutin γ, a glycoprotein previously referred to as a storage protein. J. Exp. Bot. 1995, 46, 725–728. [Google Scholar] [CrossRef]
- Monteiro, S.; Freitas, R.; Rajasekhar, B.T.; Teixeira, A.R.; Ferreira, R.B. The unique biosynthetic route from Lupinus β-conglutin gene to blad. PLoS One 2010, 5, e8542. [Google Scholar] [CrossRef]
- Duranti, M.; Consonni, A.; Magni, C.; Sessa, F.; Scarafoni, A. The major proteins of lupin seed: Characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci. Technol. 2008, 19, 624–633. [Google Scholar] [CrossRef]
- Wilson, I.B.; Zeleny, R.; Kolarich, D.; Staudacher, E.; Stroop, C.J.M.; Kamerling, J.P.; Altmann, F. Analysis of Asn-linked glycans from vegetable foodstuffs: Widespread occurrence of Lewis a, core alpha1,3-linked fucose and xylose substitutions. Glycobiology 2001, 11, 261–274. [Google Scholar] [CrossRef]
- Schiarea, S.; Arnoldi, L.; Fanelli, R.; Combarieu, E.; Chiabrando, C. In-depth glycoproteomic characterization of γ-conglutin by high-resolution accurate mass spectrometry. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Melo, T.S.; Ferreira, R.B.; Teixeira, A.N. The seed storage proteins from Lupinus albus. Phytochemistry 1994, 37, 641–648. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Melo, T.S.; Teixeira, A.N. Catabolism of the seed storage proteins from Lupinus albus. Fate of globulins during germination and seedling growth. Aust. J. Physiol. 1995, 22, 373–381. [Google Scholar] [CrossRef]
- Franco, E.; Ferreira, R.B.; Teixeira, A.R. Utilization of an improved methodology to isolate Lupinus albus conglutins in the study of their sedimentation coefficients. J. Agric. Food Chem. 1997, 45, 3908–3913. [Google Scholar] [CrossRef]
- Seabra, M.A.; Freire, J.P.B.; Ferreira, R.B.; Cunha, L.F.; Teixeira, A.R. The use of lupin seed (Lupinus albus), faba bean (Vicia faba) and cowpea (Vigna unguiculata) on the piglet weaning diet: Antigenicity and effect on growth performances. Revista Portuguesa de Zootecnia 1999, 2, 133–149. [Google Scholar]
- Wang, J.L.; Becker, J.W.; Reeke, G.N., Jr.; Edelman, G.M. A crystalline lectin from Vicia faba. J. Mol. Biol. 1974, 88, 259–262. [Google Scholar] [CrossRef]
- Allen, H.J.; Johnson, E.A.Z. Isolation and partial characterization of a lectin from Vicia faba. Biochim. Biophys. Acta 1976, 444, 374–385. [Google Scholar] [CrossRef]
- Hemperly, J.J.; Hopp, T.P.; Becker, J.W.; Cunningham, B.A. The chemical characterization of favin, a lectin isolated from Vicia faba. J. Biol. Chem. 1979, 254, 6803–6810. [Google Scholar]
- Gupta, B.K.D.; Chatterjee-Ghose, R.; Sen, A. Purification and properties of mitogenic lectins from seeds of Lathyrus sativus linn. (chickling vetch). Arch. Biochem. Biophys. 1980, 201, 137–146. [Google Scholar] [CrossRef]
- Hopp, T.P.; Hemperly, J.J.; Cuningham, B.A. Amino acid sequence and variant forms of favin, a lectin from Vicia faba. J. Biol. Chem. 1982, 257, 4479–4483. [Google Scholar]
- Lord, J.M.; Roberts, L.M.; Robertus, J.D. Ricin: Structure, mode of action, and some current applications. FASEB J. 1994, 8, 201–208. [Google Scholar]
- Bagaria, A.; Surendranath, K.; Ramagopal, U.A.; Ramakumar, S.; Karande, A.A. Structure-function analysis and insights into the reduced toxicity of Abrus precatorius agglutinin I in relation to abrin. J. Biol. Chem. 2006, 281, 34465–34474. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Lectins; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Martínez-Aragón, A.; Cavallé, C.; Frühbeck, G.; Tosar, A.; Santidrián, S.; Stewartm, J.C.; Rubio, L.; Pusztai, A. Identification and biological activity of lectins of different subunit composition isolated from Phaseolus vulgaris L var athropurpurea. J. Sci. Food Agric. 2006, 68, 375–382. [Google Scholar] [CrossRef]
- Stirpe, F. Ribosome-inactivating proteins. In Molecular Neurosurgery with Targeted Toxins; Wiley, R.G., Lappi, D.A., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2005. [Google Scholar]
- Qureshi, I.A.; Sethi, D.K.; Salunke, D.M. Purification, identification and preliminary crystallographic studies of an allergenic protein from Lathyrus sativus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 869–872. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Franco, E.; Teixeira, A.R. Calcium and magnesium-dependent aggregation of legume seed storage proteins. J. Agric. Food Chem. 1999, 47, 3009–3015. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Santos, C.N.; Ferreira, R.B.; Teixeira, A.R. Seed proteins of Lupinus mutabilis. J. Agric. Food Chem. 1997, 45, 3821–3825. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Neuhoff, V.; Arold, N.; Taube, D.; Ehrhardt, W. Improved staining of protein in PAG including IEF gels with clear background at nanogram sensitivity using Coomassie Brillant Blue G-250 and R-250. Electrophoresis 1988, 9, 255–262. [Google Scholar] [CrossRef]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.C.; Monteiro, S.V.; Carrapiço, B.M.; Ferreira, R.B. Are Vicilins Another Major Class of Legume Lectins? Molecules 2014, 19, 20350-20373. https://doi.org/10.3390/molecules191220350
Ribeiro AC, Monteiro SV, Carrapiço BM, Ferreira RB. Are Vicilins Another Major Class of Legume Lectins? Molecules. 2014; 19(12):20350-20373. https://doi.org/10.3390/molecules191220350
Chicago/Turabian StyleRibeiro, Ana C., Sara V. Monteiro, Belmira M. Carrapiço, and Ricardo B. Ferreira. 2014. "Are Vicilins Another Major Class of Legume Lectins?" Molecules 19, no. 12: 20350-20373. https://doi.org/10.3390/molecules191220350