Alteration of N-glycans and Expression of Their Related Glycogenes in the Epithelial-Mesenchymal Transition of HCV29 Bladder Epithelial Cells
Abstract
:1. Introduction
2. Results and Discussion
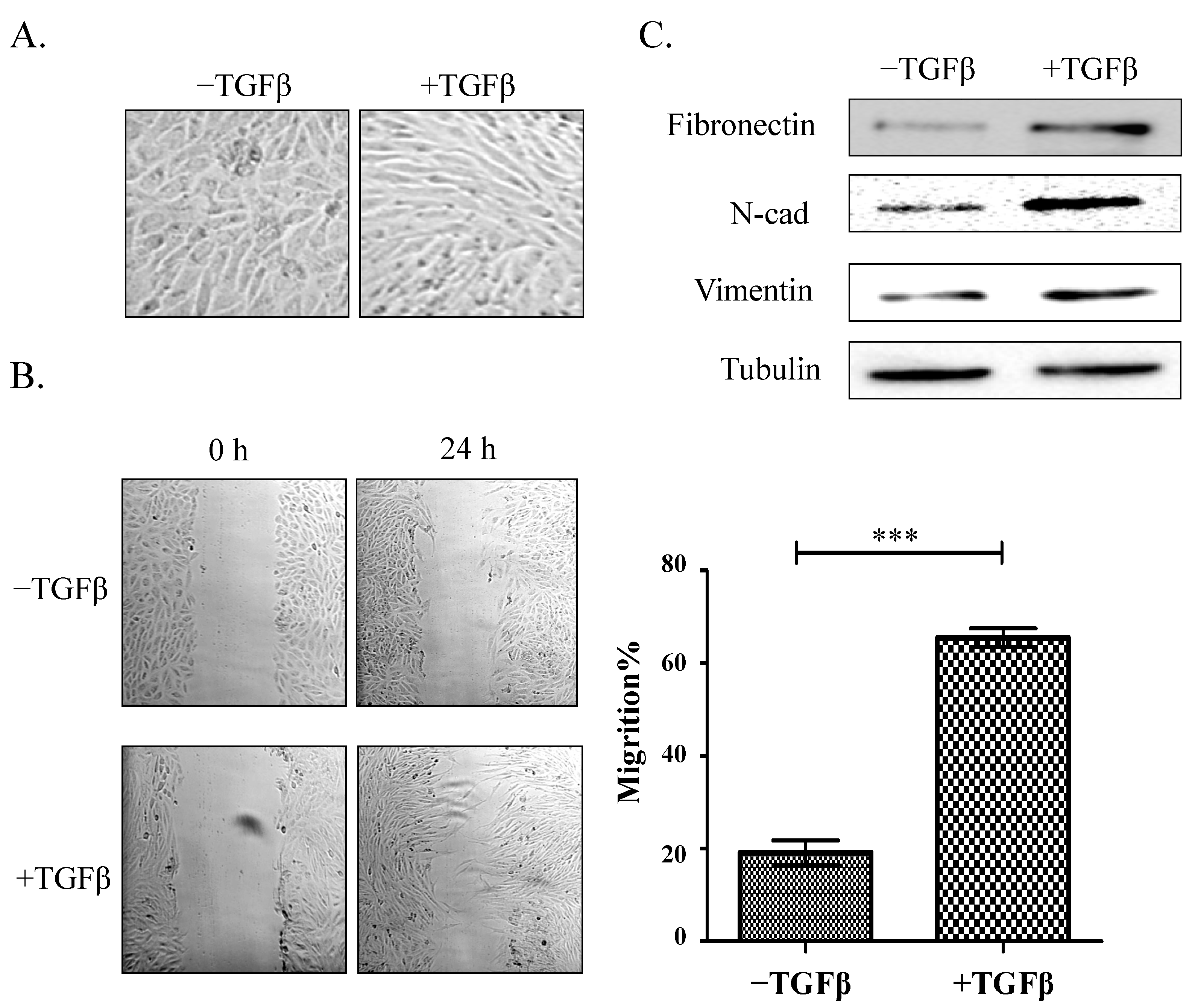
2.1. Establishment of a Model of TGFβ-Induced EMT
2.2. Glycosylation Analysis Using a Lectin Microarray during EMT in HCV29 Cells
| Lectin | Fold Change | Specificity |
|---|---|---|
| Up-regulated | ||
| UEA-I | 2.78107 | Fucα1-2Galβ1-4Glc(NAc) |
| BS-I | 2.73611 | α-Gal and α-GalNAc |
| MAL-II | 1.88948 | Sia2-3Galβ1-4Glc(NAc) |
| RCA120 | 1.63778 | β-Gal |
| PWM | 1.63695 | GlcNAc |
| Down-regulated | ||
| PNA | 0.64744 | Galβ1-3GalNAcα-Ser/Thr(T) |
| GNA | 0.64599 | Terminal α-1,3 mannose |
| SNA | 0.62468 | Sia2-6Galβ1-4Glc(NAc) |
| NPA | 0.61967 | Non-substituted α-1,6Man |
| PTL-II | 0.32613 | Gal |
| DBA | 0.25435 | αGalNAc,GalNAcα-Ser/Thr (Tn) and αGal |
| PHA-E + L | 0.2388 | Bisecting GlcNAc and biantennary N-glycans and tetra-antennary complex-type N-glycans |
| DSA | 0.21933 | GlcNAc |
| PTL-I | 0.0513 | αGalNAc and Gal |
| GSL-I | 0.00542 | αGalNAc,GalNAcα-Ser/Thr (Tn) and αGal |

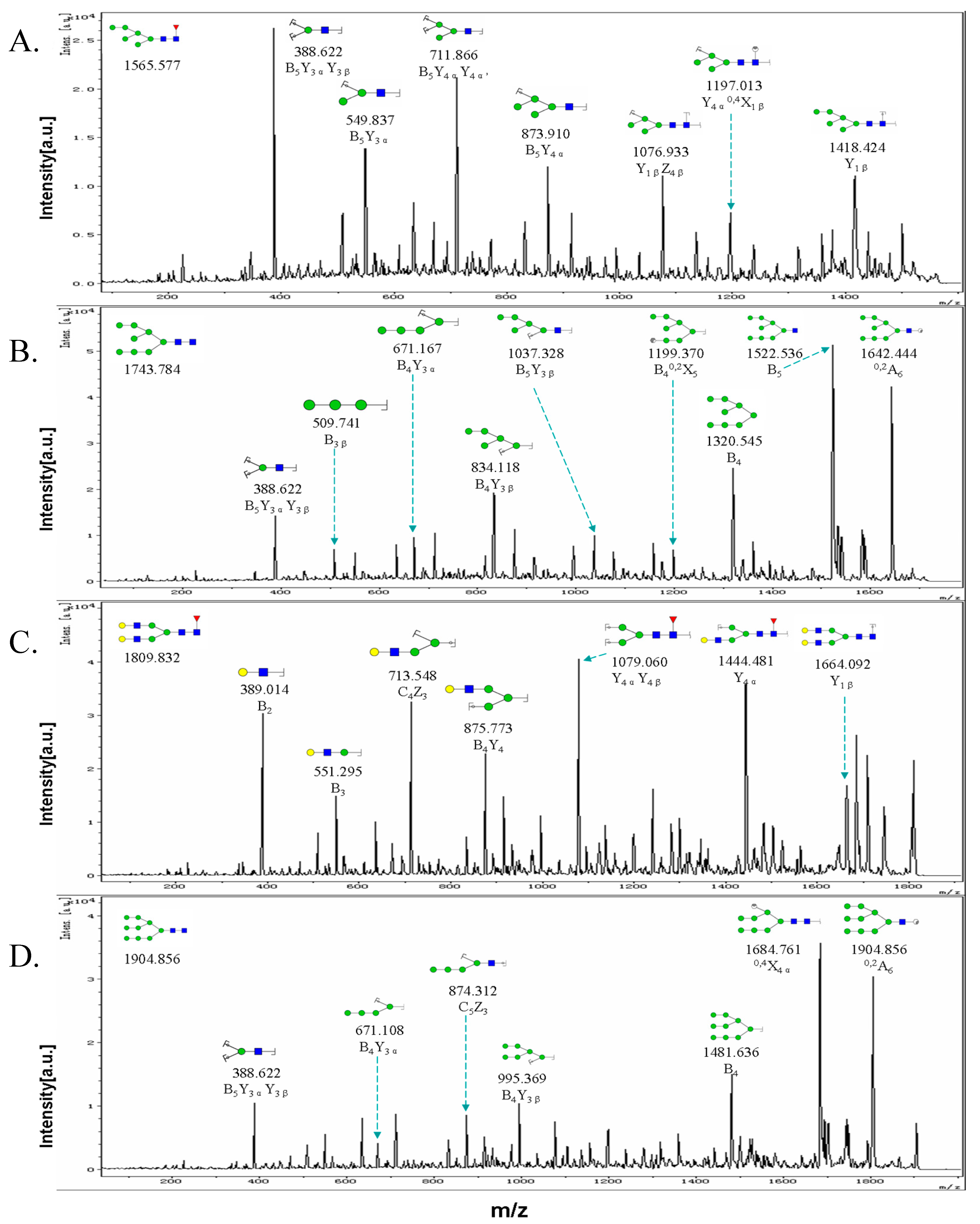
2.3. MALDI-TOF-MS Analysis of N-Glycan Profiles during EMT
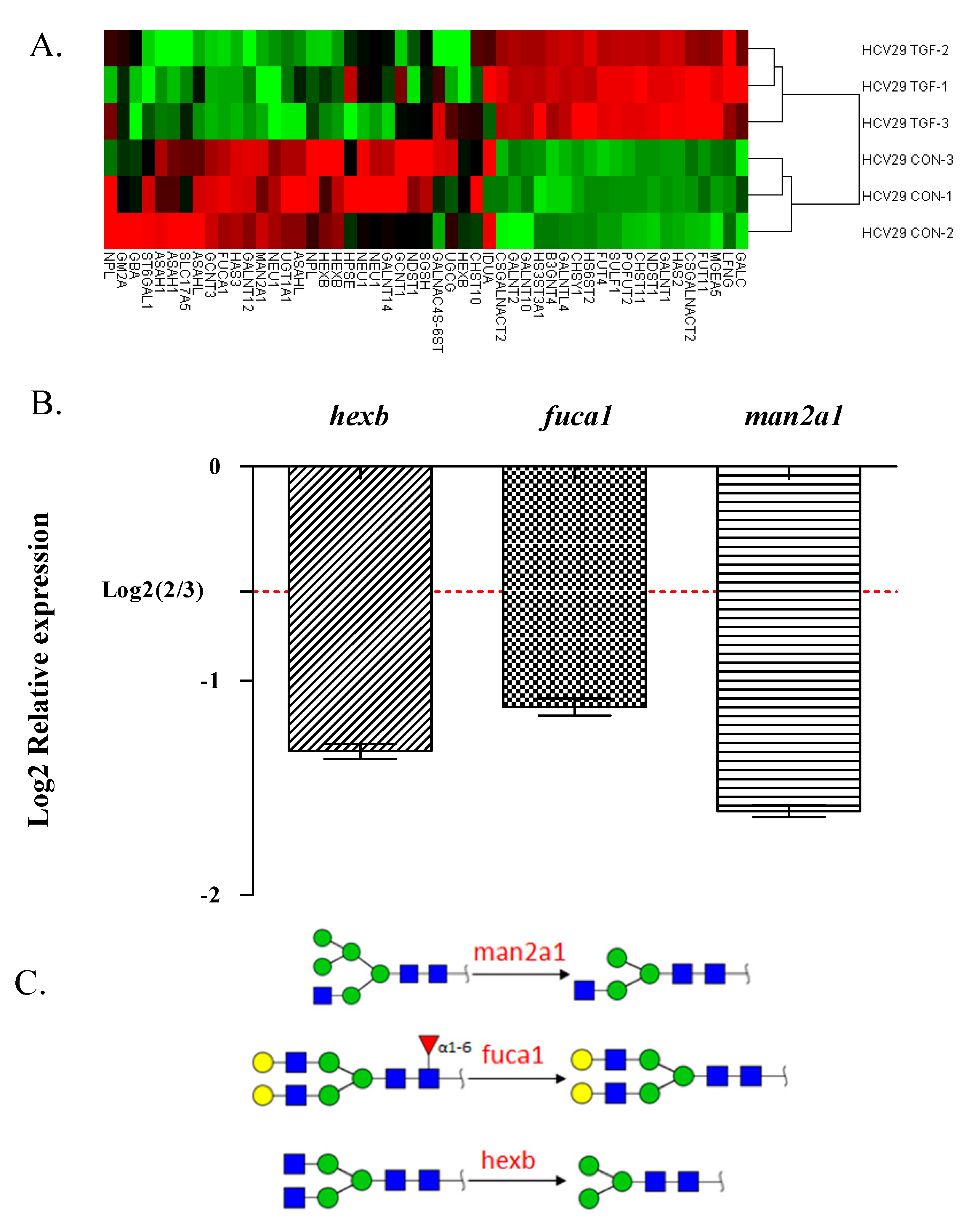
2.4. Glycogene Expression by Glycogene Microarray Analysis of TGFβ-Treated Cells
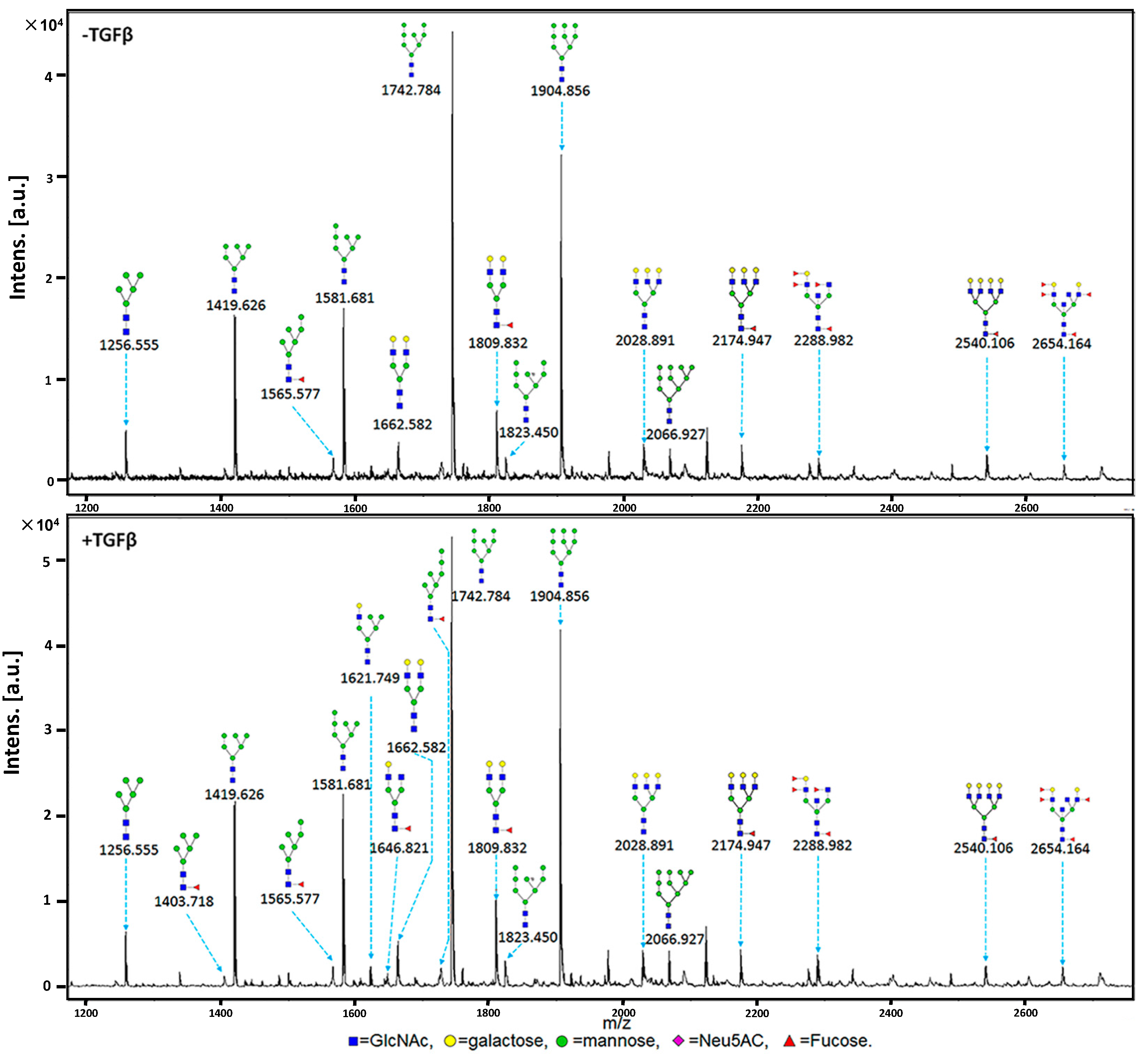
| NO. | Calculated (m/z) | Experimental (m/z) | Glycan Structure | Relative Intensity | |||
|---|---|---|---|---|---|---|---|
| Control | TGFβ-Treated | ||||||
| Average | CV(%) | Average | CV(%) | ||||
| 1 | 1079.377 | 1079.487 |  | ND * | ND | 1.50 | 0.47 |
| 2 | 1257.423 | 1256.555 |  | 6.00 | 0.73 | 11.00 | 0.55 |
| 3 | 1403.481 | 1403.718 |  | ND | ND | 1.33 | 0.43 |
| 4 | 1419.476 | 1419.626 |  | 38.67 | 0.08 | 43.67 | 0.23 |
| 5 | 1565.533 | 1565.577 |  | 3.33 | 0.17 | 3.33 | 0.17 |
| 6 | 1581.528 | 1580.681 |  | 38.33 | 0.04 | 43.33 | 0.09 |
| 7 | 1622.555 | 1621.749 |  | ND | ND | 3.33 | 0.62 |
| 8 | 1647.587 | 1646.821 |  | ND | ND | 2.00 | 0 |
| 9 | 1663.581 | 1662.582 |  | 5.33 | 0.216 | 5.67 | 0.10 |
| 10 | 1727.586 | 1726.976 |  | ND | ND | 1.33 | 0.43 |
| 11 | 1743.581 | 1742.784 |  | 100.00 | 0 | 100.00 | 0 |
| 12 | 1809.639 | 1809.832 |  | 8.33 | 0.55 | 11.33 | 0.57 |
| 13 | 1823.547 | 1823.450 |  | 4.00 | 0 | 4.00 | 0.25 |
| 14 | 1905.634 | 1904.856 |  | 64.00 | 0.07 | 69.67 | 0.11 |
| 15 | 2028.713 | 2028.891 |  | 5.00 | 0.56 | 4.00 | 0.35 |
| 16 | 2067.687 | 2066.927 |  | 3.67 | 0.42 | 5.00 | 0.35 |
| 17 | 2174.772 | 2174.947 |  | 4.00 | 0.66 | 3.67 | 0.69 |
| 18 | 2287.819 | 2288.982 |  | 3.00 | 0 | 4.00 | 0.35 |
| 19 | 2539.904 | 2540.106 |  | 2.67 | 0.57 | 2.00 | 0.5 |
| 20 | 2652.951 | 2654.164 |  | 2.00 | 0 | 2.50 | 0.28 |
| Glycan Type | Control | TGFβ-Treated Cells |
|---|---|---|
| High-mannose-type | 53.3% | 55.0% |
| Hybrid-type | 0% | 5.0% |
| Complex-type | 46.7% | 40.0% |
| Biantennary | 20.0% | 20.0% |
| Tri- and Tetra-antennary | 26.7% | 20.0% |
| Fucosylated | 40.0% | 50.0% |
| Gene Name | Genebank Acc. | Description | Fold Change |
|---|---|---|---|
| st6gal1 | NM_173216 | The encoded protein is a type II membrane protein that catalyzes the transfer of sialic acid from CMP-sialic acid to galactose-containing substrates. | 0.61 |
| neu1 | NM_000434 | The encoded protein is a lysosomal enzyme that cleaves terminal sialic acid residues from substrates such as glycoproteins and glycolipids. | 0.54 |
| hexb | NM_000521 | Hexosaminidase B is the β-subunit of the lysosomal enzyme β-hexosaminidase that, together with the cofactor GM2 activator protein, catalyzes the degradation of ganglioside GM2 and other molecules containing terminal N-acetyl hexosamines. | 0.54 |
| man2a1 | NM_002372 | The encoded protein is a member of family 38 of the glycosyl hydrolases. | 0.49 |
| fuca1 | NM_000147 | The encoded protein is a lysosomal enzyme involved in the degradation of fucose-containing glycoproteins and glycolipids. | 0.47 |
3. Experimental Section
3.1. Cell Line and Culture
3.2. Antibodies and Reagents
3.3. Total Protein Extraction and Western Blot Analysis
3.4. Wound Assay
3.5. Lectin Microarray Analysis and Data Analysis
3.6. Lectin Histochemistry
3.7. Release and Purification of N-Glycans
3.8. MALDI-TOF/TOF-MS Analysis of N-Glycans
3.9. Glycogene Microarray Analysis
3.10. Real-Time RT-PCR
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cummings, R.D. The repertoire of glycan determinants in the human glycome. Mol. Biosyst. 2009, 5, 1087–1104. [Google Scholar] [CrossRef] [PubMed]
- Zaia, J. Mass spectrometry and the emerging field of glycomics. Chem. Biol. 2008, 15, 881–892. [Google Scholar] [CrossRef] [PubMed]
- North, S.J.; Hitchen, P.G.; Haslam, S.M.; Dell, A. Mass spectrometry in the analysis of N-linked and O-linked glycans. Curr. Opin. Struct. Biol. 2009, 19, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, L.; Mahal, L.K. Glycomic analysis: An array of technologies. ACS Chem. Biol. 2009, 4, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Drake, R.R.; Schwegler, E.E.; Malik, G.; Diaz, J.; Block, T.; Mehta, A.; Semmes, O.J. Lectin capture strategies combined with mass spectrometry for the discovery of serum glycoprotein biomarkers. Mol. Cell. Proteomics 2006, 5, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Lu, Z.; Fu, Y.; Wang, H.P.; Wang, L.H.; Chi, H.; Yuan, Z.F.; Zheng, Z.B.; Song, L.N.; Han, H.H.; et al. A strategy for precise and large scale identification of core fucosylated glycoproteins. Mol. Cell. Proteomics 2009, 8, 913–923. [Google Scholar]
- Kaji, H.; Saito, H.; Yamauchi, Y.; Shinkawa, T.; Taoka, M.; Hirabayashi, J.; Kasai, K.-I.; Takahashi, N.; Isobe, T. Lectin affinity capture, isotope-coded tagging and mass spectrometry to identify N-linked glycoproteins. Nat. Biotechnol. 2003, 21, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Uchiyama, N.; Koseki-Kuno, S.; Ebe, Y.; Takashima, S.; Yamada, M.; Hirabayashi, J. Evanescent-field fluorescence-assisted lectin microarray: A new strategy for glycan profiling. Nat. Methods 2005, 2, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, I.; Nakanisi, T.; Furuta, M.; Ando, E.; Nishimura, O. Direct matrix-assisted laser desorption/ionization time-of-flight mass spectrometric identification of proteins on membrane detected by western blotting and lectin blotting. J. Proteome Res. 2005, 4, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Kumada, Y.; Ohigashi, Y.; Emori, Y.; Imamura, K.; Omura, Y.; Kishimoto, M. Improved lectin ELISA for glycosylation analysis of biomarkers using Ps-tag-fused single-chain Fv. J. Immunol. Methods 2012, 385, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Hagisawa, S.; Yoshikawa, K.; Tezuka, F.; Kaku, M.; Ohyama, C. Predictive value of N-acetylglucosaminyltransferase-V for superficial bladder cancer recurrence. J. Urol. 2006, 175, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, H.; Takahashi, T.; Nakagawa, H.; Nishimura, S.; Arai, Y.; Horikawa, Y.; Habuchi, T.; Miyoshi, E.; Kyan, A.; Hagisawa, S.; et al. N-acetylglucosaminyltransferase V and beta1–6 branching N-linked oligosaccharides are associated with good prognosis of patients with bladder cancer. Clin. Cancer Res. 2006, 12, 2506–2511. [Google Scholar]
- Videira, P.A.; Correia, M.; Malagolini, N.; Crespo, H.J.; Ligeiro, D.; Calais, F.M.; Trindade, H.; Dall’Olio, F. St3gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer 2009. [Google Scholar] [CrossRef]
- Furukawa, K.; Takamiya, K.; Okada, M.; Inoue, M.; Fukumoto, S.; Furukawa, K. Novel functions of complex carbohydrates elucidated by the mutant mice of glycosyltransferase genes. Biochim. Biophys. Acta 2001, 1525, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996, 56, 2237–2244. [Google Scholar]
- Hakomori, S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc. Natl. Acad. Sci. USA 2002, 99, 10231–10233. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.S.; Dennis, J.W. N-glycans in cancer progression. Glycobiology 2008, 18, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.E.; Koba, W.R.; Fine, E.J.; Giricz, O.; Kenny, P.A.; Stanley, P. Bisected, complex N-glycans and galectins in mouse mammary tumor progression and human breast cancer. Glycobiology 2013, 23, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Quaggin, S.E.; Kapus, A. Scar wars: Mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int. 2011, 80, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. Emt: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Handa, K.; Hakomori, S. Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proc. Natl. Acad. Sci. USA 2009, 106, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.; Dennis, J.W. Mgat5 and Pten interact to regulate cell growth and polarity. Glycobiology 2007, 17, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Isaji, T.; Lu, Y.; Gu, W.; Kondo, M.; Fukuda, T.; Du, Y.; Gu, J. Roles of N-acetylglucosaminyltransferase III in epithelial-to-mesenchymal transition induced by transforming growth factor β1 (TGF-β1) in epithelial cell lines. J. Biol. Chem. 2012, 287, 16563–16574. [Google Scholar] [CrossRef] [PubMed]
- Freire-de-Lima, L.; Gelfenbeyn, K.; Ding, Y.; Mandel, U.; Clausen, H.; Handa, K.; Hakomori, S. Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process. Proc. Natl. Acad. Sci. USA 2011, 108, 17690–17695. [Google Scholar] [CrossRef] [PubMed]
- Villar, E.; Calvo, P.; Cabezas, J.A. Comparative studies on blood serum alpha-l-fucosidases from several mammalian species. Comp. Biochem. Phys. B 1978, 60, 459–461. [Google Scholar] [CrossRef]
- Przybylo, M.; Hoja-Lukowicz, D.; Litynska, A.; Laidler, P. Different glycosylation of cadherins from human bladder non-malignant and cancer cell lines. Cancer Cell Int. 2002. [Google Scholar] [CrossRef]
- Litynska, A.; Pochec, E.; Hoja-Lukowicz, D.; Kremser, E.; Laidler, P.; Amoresano, A.; Monti, C. The structure of the oligosaccharides of alpha3beta1 integrin from human ureter epithelium (HCV29) cell line. Acta Biochim. Pol. 2002, 49, 491–500. [Google Scholar] [PubMed]
- Przybylo, M.; Litynska, A.; Pochec, E. Different adhesion and migration properties of human HCV29 non-malignant urothelial and T24 bladder cancer cells: Role of glycosylation. Biochimie 2005, 87, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Lu, W.; Li, X.; Yang, G.; Guo, J.; Yu, H.; Li, Z.; Guan, F. Altered N-glycan expression profile in epithelial-to-mesenchymal transition of NMuMG cells revealed by an integrated strategy using mass spectrometry and glycogene and lectin microarray analysis. J. Proteome Res. 2014, 13, 2783–2795. [Google Scholar] [CrossRef]
- Goldman, R.; Ressom, H.W.; Varghese, R.S.; Goldman, L.; Bascug, G.; Loffredo, C.A.; Abdel-Hamid, M.; Gouda, I.; Ezzat, S.; Kyselova, Z.; et al. Detection of hepatocellular carcinoma using glycomic analysis. Clin. Cancer Res. 2009, 15, 1808–1813. [Google Scholar]
- Zhao, Y.Y.; Takahashi, M.; Gu, J.G.; Miyoshi, E.; Matsumoto, A.; Kitazume, S.; Taniguchi, N. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008, 99, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Mechref, Y.; Novotny, M.V.; Krishnan, C. Structural characterization of oligosaccharides using MALDI-TOF/TOF tandem mass spectrometry. Anal. Chem. 2003, 75, 4895–4903. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Touster, O. Biosynthesis and modification of golgi mannosidase II in Hela and 33 cells. J. Biol. Chem. 1985, 260, 6654–6662. [Google Scholar] [PubMed]
- Moremen, K.W.; Touster, O.; Robbins, P.W. Novel purification of the catalytic domain of golgi alpha-mannosidase II. Characterization and comparison with the intact enzyme. J. Biol. Chem. 1991, 266, 16876–16885. [Google Scholar]
- Moremen, K.W.; Robbins, P.W. Isolation, characterization, and expression of cDNAs encoding murine alpha-mannosidase II, a golgi enzyme that controls conversion of high mannose to complex N-glycans. J. Cell Biol. 1991, 115, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Misago, M.; Liao, Y.F.; Kudo, S.; Eto, S.; Mattei, M.G.; Moremen, K.W.; Fukuda, M.N. Molecular cloning and expression of cDNAs encoding human alpha-mannosidase II and a previously unrecognized alpha-mannosidase IIx isozyme. Proc. Natl. Acad. Sci. USA 1995, 92, 11766–11770. [Google Scholar] [CrossRef] [PubMed]
- Schachter, H. Congenital disorders involving defective N-glycosylation of proteins. Cell. Mol. Life Sci. 2001, 58, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W. Golgi alpha-mannosidase II deficiency in vertebrate systems: Implications for asparagine-linked oligosaccharide processing in mammals. Biochim. Biophys. Acta 2002, 1573, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Chui, D.; Sellakumar, G.; Green, R.; Sutton-Smith, M.; McQuistan, T.; Marek, K.; Morris, H.; Dell, A.; Marth, J. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc. Natl. Acad. Sci. USA 2001, 98, 1142–1147. [Google Scholar] [CrossRef]
- Johnson, S.W.; Alhadeff, J.A. Mammalian alpha-l-fucosidases. Comp. Biochem. Phys. B 1991, 99, 479–488. [Google Scholar]
- Misonou, Y.; Shida, K.; Korekane, H.; Seki, Y.; Noura, S.; Ohue, M.; Miyamoto, Y. Comprehensive clinico-glycomic study of 16 colorectal cancer specimens: Elucidation of aberrant glycosylation and its mechanistic causes in colorectal cancer cells. J. Proteome Res. 2009, 8, 2990–3005. [Google Scholar] [CrossRef] [PubMed]
- Muinelo-Romay, L.; Gil-Martin, E.; Fernandez-Briera, A. Alpha(1,2)fucosylation in human colorectal carcinoma. Oncol. Lett. 2010, 1, 361–366. [Google Scholar] [PubMed]
- Straus, A.H.; Carter, W.G.; Wayner, E.A.; Hakomori, S. Mechanism of fibronectin-mediated cell migration: Dependence or independence of cell migration susceptibility on RGDS-directed receptor (integrin). Exp. Cell Res. 1989, 183, 126–139. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, M.; Qin, Y.; Zhong, Y.; Yan, H.; Wang, Q.; Bian, H.; Li, Z. Analysis of glycan-related genes expression and glycan profiles in mice with liver fibrosis. J. Proteome Res. 2012, 11, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhong, Y.; Dang, L.; Zhu, M.; Yu, H.; Chen, W.; Cui, J.; Bian, H.; Li, Z. Alteration of protein glycosylation in human hepatic stellate cells activated with transforming growth factor-β1. J. Proteome Res. 2012, 75, 4114–4123. [Google Scholar] [CrossRef]
- Yang, G.; Cui, T.; Wang, Y.; Sun, S.; Ma, T.; Wang, T.; Chen, Q.; Li, Z. Selective isolation and analysis of glycoprotein fractions and their glycomes from hepatocellular carcinoma sera. Proteomics 2013, 13, 1481–1498. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. 2004. [Google Scholar] [CrossRef]
- Guan, F.; Schaffer, L.; Handa, K.; Hakomori, S. Functional role of gangliotetraosylceramide in epithelial-to-mesenchymal transition process induced by hypoxia and by TGF-β. FASEB J. 2010, 24, 4889–4903. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Li, X.; Tan, Z.; Lu, W.; Yang, G.; Guan, F. Alteration of N-glycans and Expression of Their Related Glycogenes in the Epithelial-Mesenchymal Transition of HCV29 Bladder Epithelial Cells. Molecules 2014, 19, 20073-20090. https://doi.org/10.3390/molecules191220073
Guo J, Li X, Tan Z, Lu W, Yang G, Guan F. Alteration of N-glycans and Expression of Their Related Glycogenes in the Epithelial-Mesenchymal Transition of HCV29 Bladder Epithelial Cells. Molecules. 2014; 19(12):20073-20090. https://doi.org/10.3390/molecules191220073
Chicago/Turabian StyleGuo, Jia, Xiang Li, Zengqi Tan, Wei Lu, Ganglong Yang, and Feng Guan. 2014. "Alteration of N-glycans and Expression of Their Related Glycogenes in the Epithelial-Mesenchymal Transition of HCV29 Bladder Epithelial Cells" Molecules 19, no. 12: 20073-20090. https://doi.org/10.3390/molecules191220073




