Antiprotozoal Activity of (E)-Cinnamic N-Acylhydrazone Derivatives
Abstract
:1. Introduction
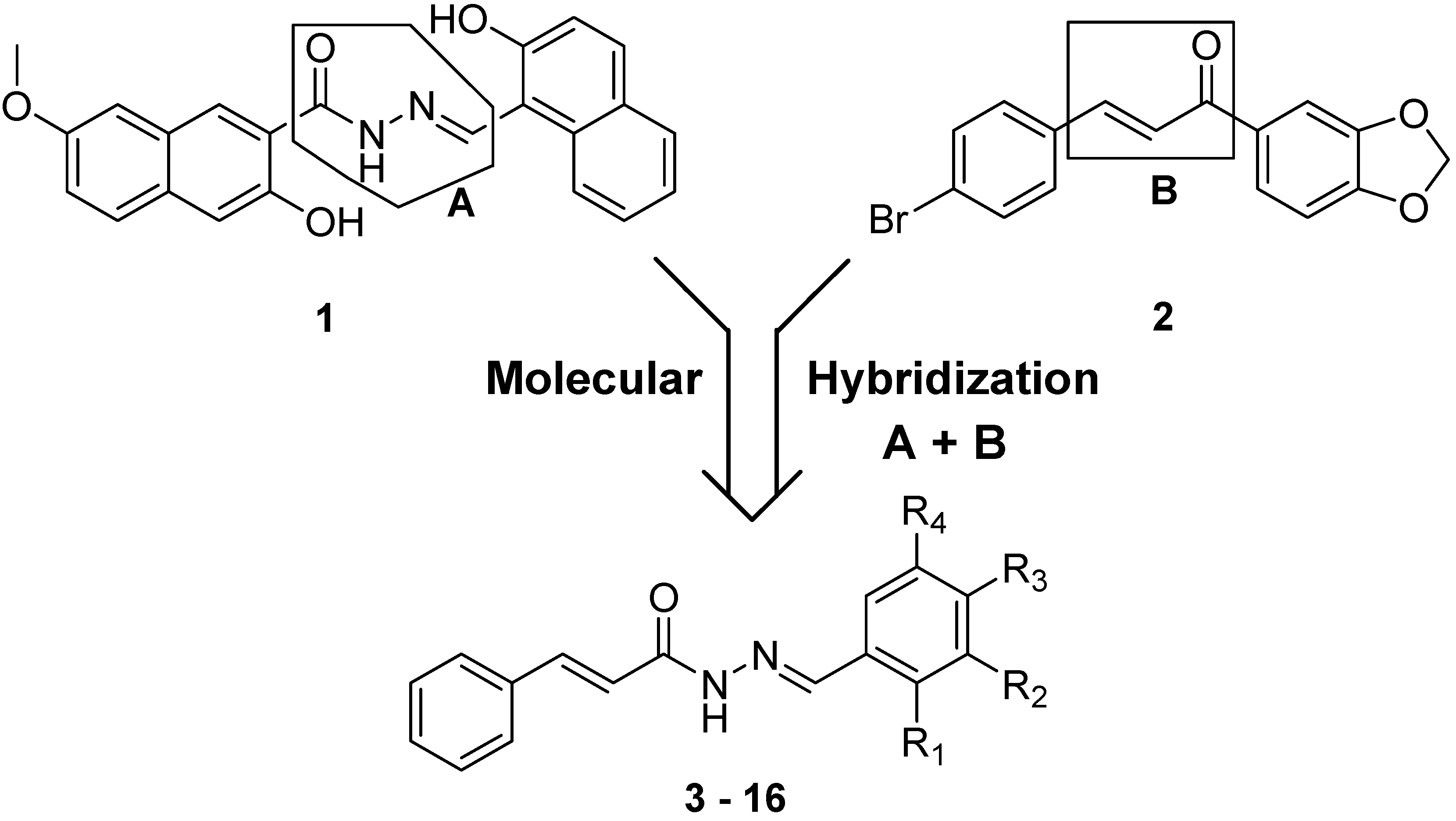
2. Results and Discussion

| Cpd. | R1 | R2 | R3 | R4 | L. donovani a | T.b. rhod. b | Cytot. L-6 Cells c | SI d | SI e |
|---|---|---|---|---|---|---|---|---|---|
| IC50 f (μM) | |||||||||
| 3 | H | OMe | OH | NO2 | 6.27 | 45.6 | >264 | >42 | >5.8 |
| 4 | H | OMe | OH | H | 21.7 | 40.8 | 95.4 | 4.4 | 2.3 |
| 5 | H | OH | OH | H | 11.7 | 1.93 | 14.0 | 1.2 | 7.3 |
| 6 | H | O-CH2-O | H | 12.5 | 89.5 | 4.3 | 0.3 | 0.05 | |
| 7 | H | OMe | OMe | OMe | 3.50 | 49.3 | 120 | 34.3 | 2.4 |
| 8 | H | H | F | H | 24.3 | 284 | >335 | >13.8 | >1.2 |
| 9 | H | H | NO2 | H | 10.6 | >300 | 255 | 24.1 | - |
| 10 | H | H | OMe | H | 13.8 | 111 | >321 | 23.3 | 2.9 |
| 11 | H | H | Cl | H | 10.8 | 121 | 65.5 | 6.0 | 0.5 |
| 12 | H | H | H | H | 43.1 | >300 | 157 | 3.6 | - |
| 13 | H | H | OH | H | 36.7 | 82.5 | 40.4 | 1.1 | 0.5 |
| 14 | H | OMe | OMe | H | 31.2 | 67.6 | >290 | >9.3 | >4.3 |
| 15 | OH | H | H | H | 3.61 | 6.72 | 14.9 | 4.1 | 2.2 |
| 16 | H | OH | OMe | H | 33.3 | 80.1 | 48.1 | 1.4 | 0.6 |
| MTS | - | - | - | - | 0.348 | - | ND | - | - |
| MLSP | - | - | - | - | - | 0.003 | ND | - | - |
| PPT | - | - | - | - | - | - | 0.006 | - | - |
3. Experimental Section
3.1. Leishmanicidal Activity
3.2. Trypanocidal Activity
3.3. Cytotoxicity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ndjonka, D.; Rapado, L.N.; Silber, A.M.; Liebau, E.; Wrenger, C. Natural Products as a Source for Treating Neglected Parasitic Diseases. Int. J. Mol. Sci. 2013, 14, 3395–3439. [Google Scholar] [CrossRef] [PubMed]
- WHO. Trypanossomiasis, Human African (Sleeping Sickness). Fact Sheet No259. Available online: http://www.who.int/mediacentre/factsheets/fs259/en/ (accessed on 3 October 2014).
- Alvar, J.; V#xE9;lez, I.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. The WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012, 7, e35671. [Google Scholar]
- World Health Organization (WHO). World Health Organization Technical Report 949: Control of the Leishmaniases; WHO: Geneva, Switzerland, 2010; pp. 1–186. [Google Scholar]
- Alvar, J.; Croft, S.; Olliaro, P. Chemotherapy in the treatment and control of leishmaniasis. Adv. Parasitol. 2006, 61, 223–274. [Google Scholar] [PubMed]
- Carvalho, S.A.; da Silva, E.F.; de Souza, M.V.N.; Lourenço, M.C.S.; Vicente, F.R. Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Kanaani, J.; Ginsburg, H. Effects of cinnamic acid derivatives on in vitro growth of Plasmodium falciparum and on the permeability of the membrane of malaria-infected erythrocytes. Antimicrob. Agents Chemother. 1992, 36, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Otero, E.; Robledo, S.M.; Diaz, S.; Carda, M.; Muñoz, D.; Paños, J.; Vélez, I.D.; Cardona, W. Synthesis and leishmanicidal activity of cinnamic esters: Structure-activity relationship. Med. Chem. Res. 2014, 23, 1378–1386. [Google Scholar] [CrossRef]
- Naz, S.; Ahmed, S.; Rasool, S.A.; Sayeed, S.A.; Siddiqi, R. Antibacterial activity directed isolation of compounds from Onosma hispidum. Microb. Res. 2006, 161, 43–48. [Google Scholar] [CrossRef]
- Ahn, B.Z.; Sok, D.E. Michael Acceptors as a Tool for Anticancer Drug Design. Curr. Pharm. Des. 1996, 2, 247–262. [Google Scholar]
- Carvalho, S.A.; Feitosa, L.O.; Soares, M.; Costa, T.E.M.M.; Henriques, M.G.; Salomão, K.; de Castro, S.L.; Kaiser, M.; Brun, R.; Wardell, J.L.; et al. Design and synthesis of new (E)-cinnamic N-acylhydrazones as potent antitrypanosomal agents. Eur. J. Med. Chem. 2012, 54, 512–521. [Google Scholar]
- Stuart, K.; Brun, R.; Croft, S.; Fairlamb, A.; Gürtler, R.E.; McKerrow, J.; Reed, S.; Tarleton, R. Kinetoplastids: Related protozoan pathogens, different diseases. J. Clin. Investig. 2008, 118, 1301–1310. [Google Scholar] [CrossRef] [Green Version]
- Borchhardt, D.M.; Mascarello, A.; Chiaradia, L.D.; Nunes, R.J.; Oliva, G.; Yunes, R.A.; Andricopulo, A.D. Biochemical Evaluation of a Series of Synthetic Chalcone and Hydrazide Derivatives as Novel Inhibitors of Cruzain from Trypanosoma cruzi. J. Braz. Chem. Soc. 2010, 21, 142–150. [Google Scholar] [CrossRef]
- Selzer, P.M.; Chen, X.; Chan, V.J.; Cheng, M.; Kenyon, G.L.; Kuntz, I.D.; Sakanari, J.A.; Cohen, F.E.; McKerrow, J.H. Leishmania major: Molecular modeling of cysteine proteases and prediction of new nonpeptide inhibitors. Exp. Parasitol. 1997, 87, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.A.; Silva, E.F.; Fraga, C.A.M.; Wardell, S.M.S.V.; Wardell, J.L.; Tiekink, E.R.T. (2E)-N'-[(E)-Benzylidene]-3-phenylprop-2-enohydrazide from synchrotron radiation. Acta Crystallogr. 2012, E68, o2255–o2256. [Google Scholar]
- Carvalho, S.A.; Silva, E.F.; Fraga, C.A.M.; Wardell, S.M.S.V.; Wardell, J.L.; Tiekink, E.R.T. (2E)-N'-[(E)-4-Chlorobenzylidene]-3-phenylprop-2-enohydrazide monohydrate. Acta Crystallogr. 2010, E66, o2410–o2411. [Google Scholar]
- Carvalho, S.A.; Silva, E.F.; Fraga, C.A.M.; Wardell, S.M.S.V.; Wardell, J.L.; Tiekink, E.R.T. (2E)-N'-[(E)-2-Hydroxybenzylidene]-3-phenylprop-2-enohydrazide. Acta Crystallogr. 2012, E68, o2253–o2254. [Google Scholar]
- Mikus, J.; Steverding, D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 2000, 48, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Baltz, T.; Baltz, D.; Giroud, C.; Crockett, J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985, 4, 1273–1277. [Google Scholar]
- Räz, B.; Iten, M.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar]
- Ifa, D.R.; Rodrigues, C.R.; Alencastro, R.B.; Fraga, C.A.M.; Barreiro, E.J. A possible molecular mechanism for the inhibition of cysteine proteases by salicylaldehyde N-acylhydrazones and related compounds. J. Mol. Struct. Theochem. 2000, 505, 11–17. [Google Scholar] [CrossRef]
- Mottram, J.C.; Coombs, G.H.; Alexander, J. Cysteine peptidases as virulence factors of Leishmania. Curr. Opin. Microbiol. 2004, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Selzer, P.M.; Pingel, S.; Hsieh, I.; Ugele, B.; Chan, V.J.; Engel, J.C.; Bogyo, M.; Russell, D.G.; Sakanari, J.A.; Mc Kerrow, J.H. Cysteine protease inhibitors as chemotherapy: Lessons from a parasite target. Proc. Natl. Acad. Sci. USA 1999, 96, 11015–11052. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Bot, C.; Kelly, J.M.; Hall, B.S. Trypanocidal activity of nitroaromatic prodrugs: Current treatments and future perspectives. Curr. Top. Med. Chem. 2011, 11, 2072–2084. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.A.; da Silva, E.F.; Santa-Rita, R.; de Castro, S.L.; Fraga, C.A.M. Synthesis and antitrypanosomal profile of new functionalized 1,3,4-thiadiazole-2-arylhydrazone derivatives, designed as non-mutagenic megazol analogues. Bioorg. Med. Chem. Lett. 2004, 14, 5967–5970. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the cinnamic N-acylhydrazone derivatives are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, S.A.; Kaiser, M.; Brun, R.; Silva, E.F.d.; Fraga, C.A.M. Antiprotozoal Activity of (E)-Cinnamic N-Acylhydrazone Derivatives. Molecules 2014, 19, 20374-20381. https://doi.org/10.3390/molecules191220374
Carvalho SA, Kaiser M, Brun R, Silva EFd, Fraga CAM. Antiprotozoal Activity of (E)-Cinnamic N-Acylhydrazone Derivatives. Molecules. 2014; 19(12):20374-20381. https://doi.org/10.3390/molecules191220374
Chicago/Turabian StyleCarvalho, Samir Aquino, Marcel Kaiser, Reto Brun, Edson Ferreira da Silva, and Carlos Alberto Manssour Fraga. 2014. "Antiprotozoal Activity of (E)-Cinnamic N-Acylhydrazone Derivatives" Molecules 19, no. 12: 20374-20381. https://doi.org/10.3390/molecules191220374




