Catalytic Asymmetric Hydrogenation of 3-Substituted Benzisoxazoles
Abstract
:1. Introduction
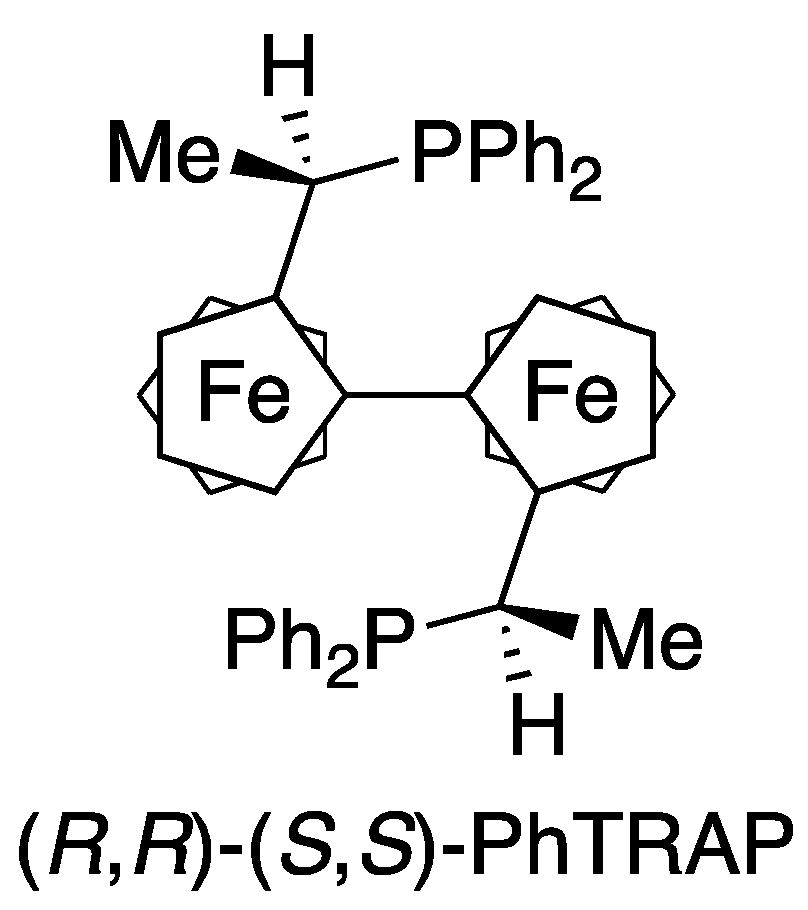
2. Results and Discussion
2.1. Optimization of Reaction Conditions
| Entry | Solvent | Acylating agent | Yield (3a), % b | Yield, % b,c | ee, % d |
|---|---|---|---|---|---|
| 1 | toluene | – | 23 | 0 (4a) | – |
| 2 | i-BuOH | – | 12 | 0 (4a) | – |
| 3 e | toluene | – | 9 | 0 (4a) | – |
| 4 e | i-BuOH | – | 13 | 0 (4a) | – |
| 5 | toluene | Boc2O | 39 | 22 (4a) | 25 (R) |
| 6 e | toluene | Boc2O | 22 | 0 (4a) | – |
| 7 | ClCH2CH2Cl | Boc2O | 63 | 19 (4a) | 30 (R) |
| 8 | CPME | Boc2O | 32 | 18 (4a) | 40 (R) |
| 9 | THF | Boc2O | 18 | 31 (4a) | 39 (R) |
| 10 | EtOAc | Boc2O | 22 | 14 (4a) | 21 (R) |
| 11 f | THF | Boc2O | 0 | >99 (93) g (4a) | 44 (R) |
| 12 | THF | Ac2O | 0 | 87 (5a) | 43 (R) |
| 13 | THF | Bz2O | 0 | 85 (6a) | 47 (R) |
| 14 | THF | Ts2O | 17 | 0 (7a) | – |
| 15 | THF | Boc-ON | 0 | 0 (4a) | – |
| 16 | CPME | Cbz-OSu | 15 | 58 (8a) | 43 (R) |
| 17 | THF | Cbz-OSu | 0 | >99 (89) g (8a) | 52 (R) |
| 18 | THF | Fmoc-OSu | 0 | >99 (93) g (9a) | 56 (R) |
| 19 f,h | THF | Cbz-OSu | 0 | 82 (8a) | 52 (R) |
| Entry | Chiral ligand | [Ru] | Yield (3a), % b | Yield (8a), % b | ee, % c |
|---|---|---|---|---|---|
| 1 d | (R,R)-(S,S)-PhTRAP | Ru(η3-methallyl)2(cod) | 0 | 0 | – |
| 2 | (R,R)-(S,S)-PhTRAP | [RuCl2(p-cymene)]2 | 25 | 47 | 53 (R) |
| 3 | (R)-(S)-BPPFA | [RuCl2(p-cymene)]2 | 28 | 6 | – |
| 4 | (R)-(S)-Josiphos | [RuCl2(p-cymene)]2 | 67 | 15 | 3 (R) |
| 5 | (R)-BINAP | [RuCl2(p-cymene)]2 | 0 | 8 | 17 (R) |
| 6 | (2S,3S)-Chiraphos | [RuCl2(p-cymene)]2 | 49 | 10 | 16 (S) |
| 7 | (2S,3S)-DIOP | [RuCl2(p-cymene)]2 | 0 | 0 | – |
| 8 | (R,R)-Me-DuPhos | [RuCl2(p-cymene)]2 | 0 | 0 | – |
2.2. Asymmetric Hydrogenations of 3-Substituted Benzisoxazoles
| Entry | R1 | R2 | Substrate (1) | Product (8) | Yield, % b | ee, % c |
|---|---|---|---|---|---|---|
| 1 | Me | H | 1b | 8b | 78 | 48 |
| 2 d | CH2CH2Ph | H | 1c | 8c | 87 | 35 |
| 3 d | i-Pr | H | 1d | 8d | 99 | 40 |
| 4 d | Ph | 6-MeO | 1e | 8e | 74 e | 55 |
| 5 | Me | 5-MeO | 1f | 8f | 82 | 54 |
| 6 | Me | 5-Me | 1g | 8g | 87 | 57 |
| 7 d | Me | 5-F | 1h | 8h | 76 | 38 |
| 8 | Me | 6-MeO | 1i | 8i | 69 | 40 |
| 9 | Me | 6-Me | 1j | 8j | 87 | 51 |
| 10 d | Me | 6-F | 1k | 8k | 82 | 23 |
| 11 d | Me | 4-MeO | 1l | 8l | 76 | 25 |
2.3. Reaction Pathway of the Asymmetric Hydrogenation of Benzisoxazoles

3. Experimental
3.1. Materials
3.2. Catalytic Asymmetric Hydrogenation of Benzisoxazoles 1
4. Conclusions
Acknowledgments
References and Notes
- Glorius, F. Asymmetric hydrogenation of aromatic compounds. Org. Biomol. Chem. 2005, 3, 4171–4175. [Google Scholar] [CrossRef]
- Zhou, Y.-G. Asymmetric hydrogenation of heteroaromatic compounds. Acc. Chem. Res. 2007, 40, 1357–1366. [Google Scholar] [CrossRef]
- Kuwano, R. Catalytic asymmetric hydrogenation of 5-membered heteroaromatics. Heterocycles 2008, 76, 909–922. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Lu, S.; Zhou, Y. Asymmetric hydrogenation of heteroarenes and arenes. Chem. Rev. 2012, 112, 2557–2590. [Google Scholar] [CrossRef]
- Wang, W.-B.; Lu, S.-M.; Yang, P.-Y.; Han, X.-W.; Zhou, Y.-G. Highly enantioselective iridium-catalyzed hydrogenation of heteroaromatic compounds, quinolines. J. Am. Chem. Soc. 2003, 125, 10536–10537. [Google Scholar]
- Lu, S.-M.; Wang, Y.-B.; Han, X.-W.; Zhou, Y.-G. Asymmetric hydrogenation of quinolines and isoquinolines activated by chloroformates. Angew. Chem. Int. Ed. 2006, 45, 2260–2263. [Google Scholar] [CrossRef]
- Rueping, M.; Antonchick, A.P.; Theissmann, T. A highly enantioselective Brønsted acid catalyzed cascade reaction: organocatalytic transfer hydrogenation of quinolines and their application in the synthesis of alkaloids. Angew. Chem. Int. Ed. 2006, 45, 3683–3686. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Z.; Wang, Z.; Wang, T.; Xu, L.; He, Y.; Fan, Q.-H.; Pan, J.; Gu, L.; Chan, A.S.C. Hydrogenation of quinolines using a recyclable phosphine-free chiral cationic ruthenium catalyst: enhancement of catalyst stability and selectivity in an ionic liquid. Angew. Chem. Int. Ed. 2008, 47, 8464–8467. [Google Scholar]
- Wang, T.; Zhuo, L.-G.; Li, Z.; Chen, F.; Ding, Z.; He, Y.; Fan, Q.-H.; Xiang, J.; Yu, Z.-X.; Chan, A.S.C. Highly enantioselective hydrogenation of quinolines using phosphine-free chiral cationic ruthenium catalysts: Scope, mechanism, and origin of enantioselectivity. J. Am. Chem. Soc. 2011, 133, 9878–9891. [Google Scholar]
- Bianchini, C.; Barbaro, P.; Scapacci, G.; Farnetti, E.; Graziani, M. Enantioselective hydrogenation of 2-methylquinoxaline to (-)-(2S)-2-methyl-1,2,3,4-tetrahydroquinoxaline by iridium catalysis. Organometallics 1998, 17, 3308–3310. [Google Scholar]
- Tang, W.; Xu, L.; Fan, Q.-H.; Wang, J.; Fan, B.; Zhou, Z.; Lam, K.-H.; Chan, A.S.C. Asymmetric hydrogenation of quinoxalines with diphosphinite ligands: a practical synthesis of enantioenriched, substituted tetrahydroquinoxalines. Angew. Chem. Int. Ed. 2009, 48, 9135–9138. [Google Scholar]
- Urban, S.; Ortega, N.; Glorius, F. Ligand-controlled highly regioselective and asymmetric hydrogenation of quinoxalines catalyzed by ruthenium N-heterocyclic carbene complexes. Angew. Chem. Int. Ed. 2011, 50, 3803–3806. [Google Scholar] [CrossRef]
- Chen, Q.-A.; Wang, D.-S.; Zhou, Y.-G.; Duan, Y.; Fan, H.-J.; Yang, Y.; Zhang, Z. Convergent asymmetric disproportionation reactions: metal/Brønsted acid relay catalysis for enantioselective reduction of quinoxalines. J. Am. Chem. Soc. 2011, 133, 6126–6129. [Google Scholar]
- Chen, Q.-A.; Gao, K.; Duan, Y.; Ye, Z.-S.; Shi, L.; Yang, Y.; Zhou, Y.-G. Dihydrophenanthridine: A new and easily regenerable NAD(P)H model for biomimetic asymmetric hydrogenation. J. Am. Chem. Soc. 2012, 134, 2442–2448. [Google Scholar] [CrossRef]
- Legault, C.Y.; Charette, A.B. Catalytic asymmetric hydrogenation of N-iminopyridinium ylides: Expedient approach to enantioenriched substituted piperidine derivatives. J. Am. Chem. Soc. 2005, 127, 8966–8967. [Google Scholar] [CrossRef]
- Rueping, M.; Antonchick, A.P. Organocatalytic enantioselective reduction of pyridines. Angew. Chem. Int. Ed. 2007, 46, 4562–4565. [Google Scholar] [CrossRef]
- Wang, X.-B.; Zeng, W.; Zhou, Y.-G. Iridium-catalyzed asymmetric hydrogenation of pyridine derivatives, 7,8-dihydro-quinolin-5(6H)-ones. Tetrahedron Lett. 2008, 49, 4922–4924. [Google Scholar] [CrossRef]
- Kaiser, S.; Smidt, S.P.; Pfaltz, A. Iridium catalysts with bicyclic pyridine-phosphinite ligands: Asymmetric hydrogenation of olefins and furan derivatives. Angew. Chem. Int. Ed. 2006, 45, 5194–5197. [Google Scholar]
- Ortega, N.; Urban, S.; Beiring, B.; Glorius, F. Ruthenium NHC catalyzed highly asymmetric hydrogenation of benzofurans. Angew. Chem. Int. Ed. 2012, 51, 1710–1713. [Google Scholar] [CrossRef]
- Sawamura, M.; Hamashima, H.; Sugawara, M.; Kuwano, R.; Ito, Y. Synthesis and structures of trans-chelating chiral diphosphine ligands bearing aromatic P-substituents, (S,S)-(R,R)- and (R,R)-(S,S)-2,2"-bis[1-(diarylphosphino)ethyl]-1,1"-biferrecenes (ArylTRAPs) and their transition metal complexes. Organometallics 1995, 14, 4549–4558. [Google Scholar] [CrossRef]
- Kuwano, R.; Sawamura, M. Rhodium–catalyzed asymmetric hydrogenation of indoles. In Catalysts for Fine Chemical Synthesis, Regio- and Stereo- Controlled Oxidations and Reductions; Roberts, S.M., Whittall, J., Eds.; John Wiley & Sons: West Sussex, UK, 2007; Volume 5, pp. 73–86. [Google Scholar]
- Kuwano, R.; Sato, K.; Kurokawa, T.; Karube, D.; Ito, Y. Catalytic asymmetric hydrogenation of heteroaromatic compounds, indoles. J. Am. Chem. Soc. 2000, 122, 7614–7615. [Google Scholar]
- Kuwano, R.; Kaneda, K.; Ito, T.; Sato, K.; Kurokawa, T.; Ito, Y. Highly enantioselective synthesis of chiral 3-substituted indolines by catalytic asymmetric hydrogenation of indoles. Org. Lett. 2004, 6, 2213–2215. [Google Scholar] [CrossRef]
- Kuwano, R.; Kashiwabara, M.; Sato, K.; Ito, T.; Kaneda, K.; Ito, Y. Catalytic asymmetric hydrogenation of indoles using a rhodium complex with a chiral bisphosphine ligand PhTRAP. Tetrahedron: Asymmetry 2006, 17, 521–535. [Google Scholar] [CrossRef]
- Kuwano, R.; Kashiwabara, M. Ruthenium-catalyzed asymmetric hydrogenation of N-Boc-indoles. Org. Lett. 2006, 8, 2653–2655. [Google Scholar] [CrossRef]
- Wang, D.-S.; Chen, Q.-A.; Li, W.; Yu, C.-B.; Zhou, Y.-G.; Zhang, X. Pd-catalyzed asymmetric hydrogenation of unprotected indoles activated by Brønsted acids. J. Am. Chem. Soc. 2010, 132, 8909–8911. [Google Scholar] [CrossRef]
- Baeza, A.; Pfaltz, A. Iridium-catalyzed asymmetric hydrogenation of N-protected indoles. Chem. Eur. J. 2010, 16, 2036–2039. [Google Scholar] [CrossRef]
- Kuwano, R.; Kashiwabara, M.; Ohsumi, M.; Kusano, H. Catalytic asymmetric hydrogenation of 2,3,5-trisubstituted pyrroles. J. Am. Chem. Soc. 2008, 130, 808–809. [Google Scholar] [CrossRef]
- Wang, D.-S.; Ye, Z.-S.; Chen, Q.-H.; Zhou, Y.-G.; Yu, C.-B.; Fan, H.-J.; Duan, Y. Highly enantioselective partial hydrogenation of simple pyrroles: A facile access to chiral 1-pyrrolines. J. Am. Chem. Soc. 2011, 133, 8866–8869. [Google Scholar]
- Kuwano, R.; Kameyama, N.; Ikeda, R. Catalytic asymmetric hydrogenation of N-Boc-imidazoles and oxazoles. J. Am. Chem. Soc. 2011, 133, 7312–7315. [Google Scholar] [CrossRef]
- Very recently, we have reported the catalytic asymmetric hydrogenation of naphthalenes: Kuwano, R.; Morioka, R.; Kashiwabara, M.; Kameyama, N. Catalytic asymmetric hydrogenation of naphthalenes. Angew. Chem. Int. Ed. 2012, 51, 4136–4139.
- Rakhshind, M.A.; Kahn, N.H. Reaction of azalactone with hydroxylamine synthesis of β-aminophenylalanine. Synth. Commun. 1978, 8, 497–510. [Google Scholar] [CrossRef]
- Basheeruddin, K.; Siddiqui, A.A.; Khan, N.H.; Saleh, S. A convenient synthesis of β-amino acid. Synth. Commun. 1979, 9, 705–712. [Google Scholar] [CrossRef]
- Yamazaki, N.; Ito, T.; Kibayashi, C. Total synthesis of the coccinellid alkaloid (–)-adalinine and the assignment of its absolute configuration. Tetrahedron Lett. 1999, 40, 739–742. [Google Scholar] [CrossRef]
- Yamazaki, N.; Ito, T.; Kibayashi, C. Asymmetric synthesis of (–)-indolizidines 167B and 209D based on stereocontrolled allylation of a chiral tricyclic N-acyl-N,O-acetal. Org. Lett. 2000, 2, 465–467. [Google Scholar] [CrossRef]
- Schwenkreis, T.; Berkessel, A. A biomimetic catalyst for the asymmetric epoxidation of unfunctionalized olefins with hydrogen peroxide. Tetrahedron Lett. 1993, 34, 4785–4788. [Google Scholar] [CrossRef]
- Palmieri, G. A practical o-hydroxybenzylamines promoted enantioselective addition of dialkylzincs to aldehydes with asymmetric amplification. Tetrahedron: Asymmetry 2000, 11, 3361–3373. [Google Scholar] [CrossRef]
- Yang, X.; Hirose, T.; Zhang, G. Catalytic enantioselective arylation of aryl aldehydes by chiral aminophenol ligands. Tetrahedron: Asymmetry 2009, 20, 415–419. [Google Scholar] [CrossRef]
- Ziyaev, R.; Irgashev, T.; Israilov, I.A.; Abdullaev, N.D.; Yunusov, M.S.; Yunusov, S.Y. Alkaloids of Ziziphus jujuba the structure of juziphine and juzirine. Chem. Nat. Comp. 1977, 13, 204–207. [Google Scholar] [CrossRef]
- Manfredi, K.P.; Blunt, J.W.; Cardellina J.H., II.; McMahon, J.B.; Pannell, L.L.; Cragg, G.M.; Boyd, M.R. Novel alkaloids from the tropical plant Ancistrocladus abbreviatus inhibit cell killing by HIV-1 and HIV-2. J. Med. Chem. 1991, 34, 3402–3405. [Google Scholar] [CrossRef]
- Bringmann, G.; Günther, C.; Saeb, W.; Mies, J.; Brun, R.; Assic, L.A. 8-O-Methyldioncophyllinol B and revised structures of other 7,6’-coupled naphthylisoquinoline alkaloids from Triphyophyllum peltatum (Dioncophyllaceae). Phytochemistry 2000, 54, 337–346. [Google Scholar]
- Bringmann, G.; Günther, C.; Saeb, W.; Mies, J.; Wickramasinghe, A.; Mudogo, V.; Brun, R. Ancistrolikokines A−C: new 5,8′-coupled naphthylisoquinoline alkaloids from Ancistrocladuslikoko. J. Nat. Prod. 2000, 63, 1333–1337. [Google Scholar] [CrossRef]
- Vaijayanthi, T.; Chadha, A. Asymmetric reduction of aryl imines using Candida parapsilosisATCC 7330. Tetrahedron: Asymmetry 2007, 19, 93–96. [Google Scholar] [CrossRef]
- Bernardinelli, G.; Fernandez, D.; Gosmini, R.; Meier, P.; Ripa, A.; Schüpfer, P.; Treptow, B.; Kündig, E.P. a-t-Butyl- and α-i-propyl-ortho-hydroxybenzylamines: Racemic synthesis/resolution and asymmetric synthesis. Chirality 2000, 12, 529–539. [Google Scholar] [CrossRef]
- Cimarelli, C.; Palmieri, G. Asymmetric reduction of enantiopure imines with zinc borohydride: Stereoselective synthesis of chiral amines. Tetrahedron: Asymmetry 2000, 11, 2555–2563. [Google Scholar] [CrossRef]
- Kündig, E.P.; Botuha, C.; Lemercier, G.; Romanens, P.; Saudan, L.; Thibault, S. Asymmetric syntheses of 2-(1-aminoethyl)phenols. Helv. Chim. Acta 2004, 87, 561–579. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Bousserouel, H.; Wang, Q.; Guéritte, F. Chiral phosphoric acid-catalyzed enantioselective transfer hydrogenation of ortho-hydroxyaryl alkyl N–H ketimines. Org. Lett. 2010, 12, 4705–4707. [Google Scholar]
- Nguyen, T.B.; Wang, Q.; Guéritte, F. Chiral phosphoric acid catalyzed enantioselective transfer hydrogenation of ortho-hydroxybenzophenone NH ketimines and applications. Chem. Eur. J. 2011, 17, 9576–9580. [Google Scholar] [CrossRef]
- Naruto, S.; Nagamoto, N.; Mizuta, H.; Yoshida, T.; Uno, H. Studies on 3-substituted 1,2-benzisoxazole derivatives. IX. Synthesis of 4-oxo-4H-benzisoxazolo[2,3-a]pyridines via dimerization of 1,2-benzisoxazole-3-acetic acids. Chem. Pharm. Bull. 1982, 30, 3418–3420. [Google Scholar] [CrossRef]
- Lepore, S.D.; Schacht, A.L.; Wiley, M.R. Preparation of 2-hydroxybenzamidines from 3-aminobenzisoxazoles. Tetrahedron Lett. 2002, 43, 8777–8779. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamamoto, K.; Kumada, M. Asymmetric catalytic hydrosilylation of ketones preparation of chiral ferrocenylphosphines as chiral ligands. Tetrahedron Lett. 1974, 15, 4405–4408. [Google Scholar] [CrossRef]
- Hayashi, T.; Mise, T.; Fukushima, M.; Kagotani, M.; Nagashima, N.; Hamada, Y.; Matsumoto, A.; Kawakami, S.; Konishi, M.; Yamamoto, K.; Kumada, M. Asymmetric synthesis catalyzed by chiral ferrocenylphosphine–transition metal complexes. I. preparation of chiral ferrocenylphosphines. Bull. Chem. Soc. Jpn. 1980, 53, 1138–1151. [Google Scholar] [CrossRef]
- Togni, A.; Breutel, C.; Schnyder, A.; Spindler, F.; Landert, H.; Tijani, A. A novel easily accessible chiral ferrocenyldiphosphine for highly enantioselective hydrogenation, allylic alkylation, and hydroboration reactions. J. Am. Chem. Soc. 1994, 116, 4062–4066. [Google Scholar]
- Fryzuk, M.D.; Bosnich, B. Asymmetric synthesis. Production of optically active amino acids by catalytic hydrogenation. J. Am. Chem. Soc. 1977, 99, 6262–6267. [Google Scholar] [CrossRef]
- Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. Synthesis of 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl (BINAP), an atropisomeric chiral bis(triaryl)phosphine, and its use in the rhodium(I)-catalyzed asymmetric hydrogenation of α-(acylamino)acrylic acids. J. Am. Chem. Soc. 1980, 102, 7932–7934. [Google Scholar]
- Takaya, H.; Mashima, K.; Koyano, K.; Yagi, M.; Kumobayashi, H.; Taketomi, T.; Akutagawa, S.; Noyori, R. Practical synthesis of (R)- or (S)-2,2’-bis(diarylphosphino)-1,1’-binaphthyls (BINAPs). J. Org. Chem. 1986, 51, 629–635. [Google Scholar] [CrossRef]
- Kagan, H.B.; Dang, T.-P. Asymmetric catalytic reduction with transition metal complexes. I. catalytic system of rhodium(I) with (–)-2,3-O-isopropylidene-2,3-dihydroxy-1,4-bis(diphenyl-phosphino)butane, a new chiral diphosphine. J. Am. Chem. Soc. 1972, 94, 6429–6433. [Google Scholar] [CrossRef]
- Burk, M.J. C2-symmetric bis(phospholanes) and their use in highly enantioselective hydrogenation reactions. J. Am. Chem. Soc. 1991, 113, 8518–8519. [Google Scholar] [CrossRef]
- Frankel, M.; Ladkany, D.; Gilon, C.; Wolman, Y. The preparation of alkyloxycarbonyl amino acids and their N-hydroxysuccinimide esters. Tetrahedron Lett. 1966, 7, 4765–4768. [Google Scholar] [CrossRef]
- Aimoto, S.; Shimonishi, Y. Regeneration to the native form of hen egg-white lysozyme from its protected derivatives. Bull. Chem. Soc. Jpn. 1978, 51, 205–213. [Google Scholar] [CrossRef]
- A review of the catalytic asymmetric hydrogenation of the C–N double bonds: Fleury-Brégeot, N.; de la Fuente, V.; Castillón, S.; Claver, C. Highlights of transition metal-catalyzed asymmetric hydrogenation of imines. ChemCatChem 2010, 2, 1346–1371.
- Hansen, K.B.; Rosner, T.; Kubryk, M.; Dormer, P.G.; Armstrong, J.D., III. Detection and elimination of product inhibition from the asymmetric catalytic hydrogenation of enamines. Org. Lett. 2005, 7, 4975–4938. [Google Scholar] [CrossRef]
- Chen, F.; Wang, T.; He, Y.; Ding, Z.; Li, Z.; Xu, L.; Fan, Q. Asymmetric hydrogenation of N-alkyl ketimines with phosphine-free, chiral, cationic Ru–MsDPEN catalysts. Chem. Eur. J. 2011, 17, 1109–1113. [Google Scholar]
- Marcazzan, P.; Patrick, B.O.; James, B.R. Amine products and catalyst poisoning in the homogeneous H2 hydrogenation of imines catalyzed by the [Rh(COD)(PPh3)2]PF6precursor. Organometallics 2003, 22, 1177–1179. [Google Scholar] [CrossRef]
- Marcazzan, P.; Patrick, B.O.; James, B.R. Rhodium–hydrido–benzylamine–triphenylphosphine complexes: Solid-state and solution structures and implications in catalyzed imine hydrogenation. Inorg. Chem. 2004, 43, 6838–6841. [Google Scholar] [CrossRef]
- Jain, M.; Kwon, C. 1,2-Benzisoxazole phosphorodiamidates as novel anticancer prodrugs requiring bioreductive activation. J. Med. Chem. 2003, 46, 5428–5436. [Google Scholar] [CrossRef]
- Kohler, E.P.; Bruce, W.F. The oximes of ortho hydroxy benzophenone. J. Am. Chem. Soc. 1931, 53, 1569–1574. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ikeda, R.; Kuwano, R. Catalytic Asymmetric Hydrogenation of 3-Substituted Benzisoxazoles. Molecules 2012, 17, 6901-6915. https://doi.org/10.3390/molecules17066901
Ikeda R, Kuwano R. Catalytic Asymmetric Hydrogenation of 3-Substituted Benzisoxazoles. Molecules. 2012; 17(6):6901-6915. https://doi.org/10.3390/molecules17066901
Chicago/Turabian StyleIkeda, Ryuhei, and Ryoichi Kuwano. 2012. "Catalytic Asymmetric Hydrogenation of 3-Substituted Benzisoxazoles" Molecules 17, no. 6: 6901-6915. https://doi.org/10.3390/molecules17066901







