Current Status of Older and New Purine Nucleoside Analogues in the Treatment of Lymphoproliferative Diseases
Abstract
:Contents
- Introduction
- Pharmacology and mechanism of action
- Clinical activity
- Side effects and tolerability
- Conclusions
1. Introduction
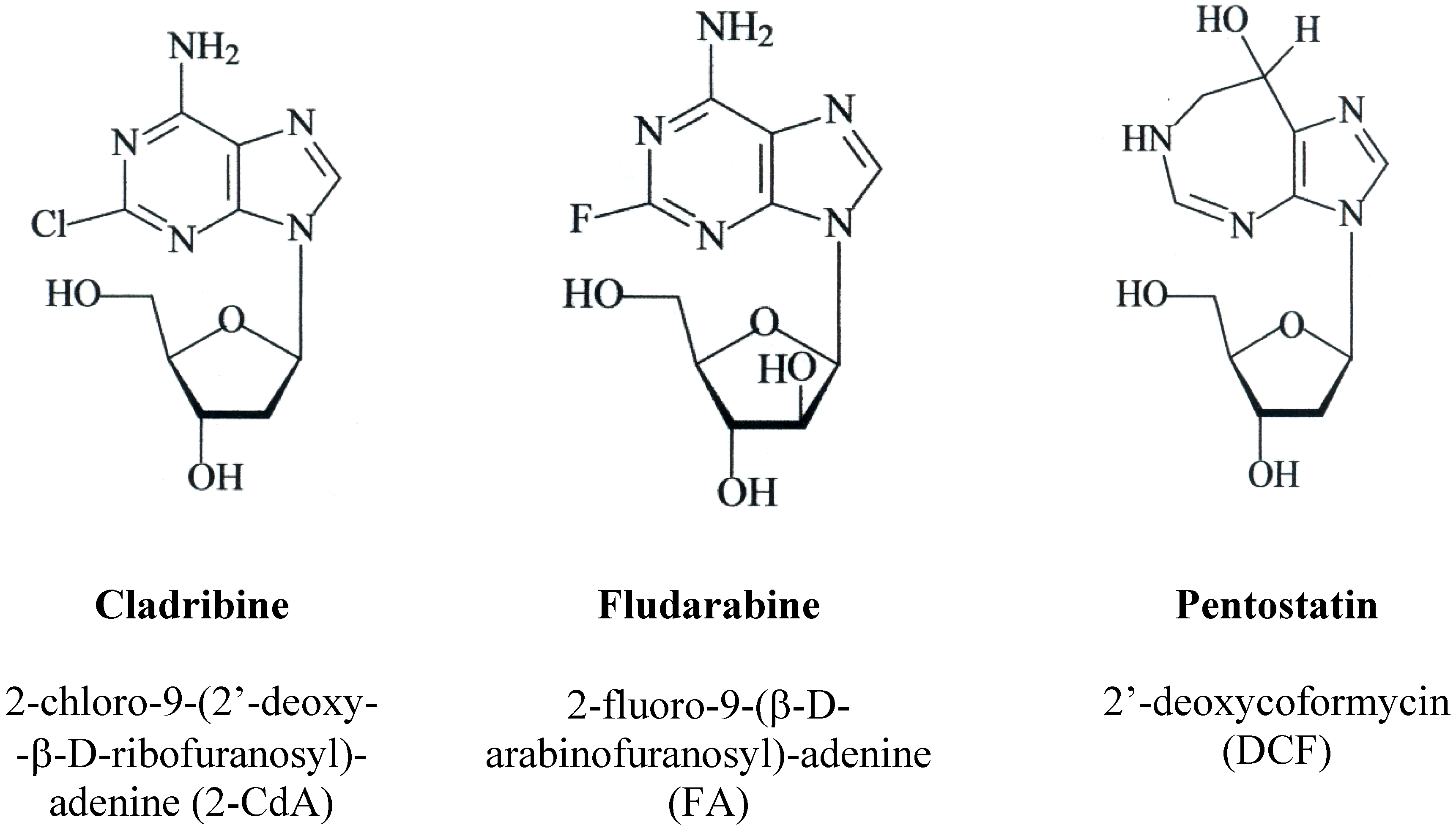

2. Pharmacology and Mechanism of Action
2.1. Fludarabine
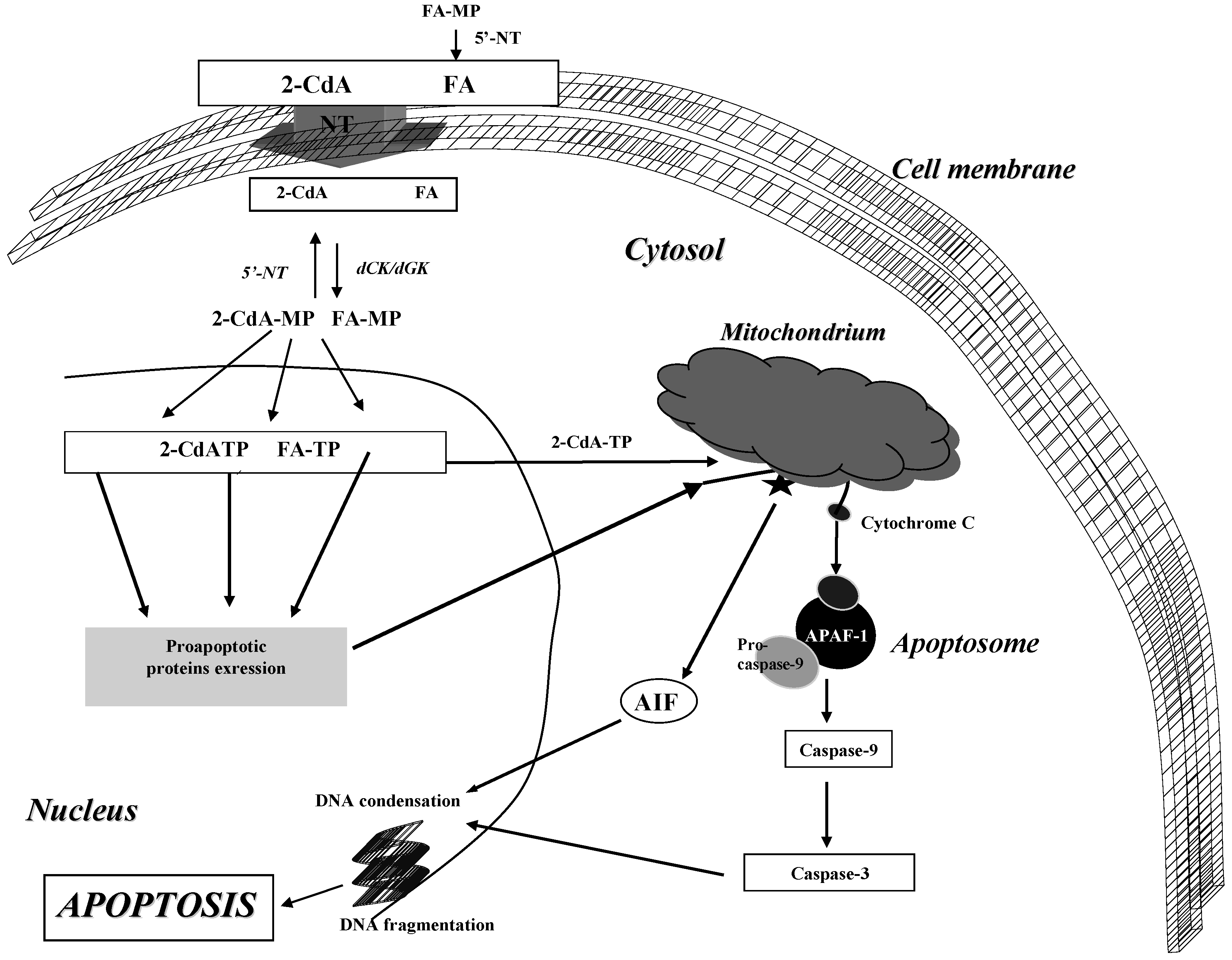
2.2. Cladribine
2.3. Pentostatin
2.4. Clofarabine

2.5. Nalarabine
2.6. Forodesine
3. Clinical activity
3.1. Hairy cell leukemia
3.2. Chronic Lymphocytic Leukemia
3.3. Prolymphocytic leukemia
3.4. Acute lymphoblastic leukemia
3.5. Non-Hodgkin lymphoma
4. Side Effects and Tolerability
5. Conclusions
Acknowledgements
List of Abbreviations
| 2-CdA- | 2-chloro-2’-deoxyadenosine; 2-chloro-9-(2’-deoxy-β-d-ribofuranosyl)-adenine; cladribine |
| 5’-NT- | 5’-nucleotidase |
| AA- | aplastic anemia |
| ADA- | adenine deaminase |
| Ado- | adenosine |
| AIF- | apoptosis-inducing factor |
| AIHA- | autoimmune hemolytic anemia |
| ALL- | acute lymphoblastic leukemia |
| AML- | acute myeloid leukemia |
| APAF-1- | apoptotic protease activating factor |
| Ara-C– | 9-β-d-arabinofuranosylcytosine; cytarabine |
| Ara-G- | 9-β-d-arabinofuranosylguanine; nelarabine |
| AUC- | area under the concentration-time curve |
| BCX-1777- | immucillin H; forodesine |
| CAFdA- | 2-chloro-2’-fluoro-2’-deoxyadenosine; 2-chloro-9-(2’-deoxy-2’-fluoro-β-d-arabinofuranosyl)-adenine; clofarabine |
| Chl- | chlorambucil |
| CLL- | chronic lymphocytic leukemia |
| Cmax- | maximum plasma concentration |
| CML-BP- | chronic myelogenous leukemia blast phase |
| CR- | complete response |
| CTCL- | cutaneous T-cell lymphoma |
| CY- | cyclophosphamide |
| dAdo- | deoxyadenosine |
| DCF- | 2’-deoxyocoformycin; pentostatin |
| dCK- | deoxycytidine kinase |
| dGK- | deoxyguanine kinase |
| dGuo- | 2’-deoxyguanosine |
| DLBCL- | diffusse large B-cell lymphoma |
| DLT- | dose limiting toxicity |
| DNMT1-DNA | methylotransferase |
| DNR- | daunorubicin |
| FA- | 2-fluoroadenosine; 2-fluoro-9-(β-d-arabinofuranosyl)-adenine; fludarabine |
| FAMP- | fludarabine monophosphate |
| FC- | fludarabine + cyclophosphamide |
| FCM- | fludarabine + cyclophosphamide + mitoxantrone |
| FL- | follicular lymphoma |
| FM- | fludarabine + mitoxantrone |
| FMC- | fludarabine + mitoxantrone + cyclophosphamide |
| G-CSF- | granulocyte colony stimulating factor |
| HCL- | hairy cell leukaemia |
| hCNT- | human concentrative nucleoside transporter |
| hENT- | human equilibrative nucleoside transporter |
| LG-NHL- | low grade non-Hodgkin’s lymphoma |
| MALT- | mucosa associated lymphoid tissue lymphoma |
| MCL- | mantle cell lymphoma |
| MRD- | minimal residual disease |
| MDS- | myelodysplastic syndrome |
| MIT- | mitoxantrone |
| MTD- | maximum tolerated dose |
| NHL- | non-Hodgkin’s lymphoma |
| OR– | overall response |
| OS- | overall survival |
| PARP- | poly ADP-ribose polymerase |
| PFS- | progression free survival |
| PLL- | prolymphocytic leukemia |
| PNA- | purine nucleoside analogues |
| PNP- | purine nucleoside phosphorylase |
| PR- | partial response |
| RCC- | rituximab + cladribine + cyclophosphamide |
| R-FC- | rituximab + fludarabine + cyclophosphamide |
| RR- | ribonucleotide reductase |
| SLL- | small lymphocytic lymphoma |
| T1/2- | half life time |
| Vd- | value of distribution |
| WM- | Waldenström macroglobulinemia |
References
- Tallman, M.S.; Hakimian, D.; Variakojis, D.; Koslow, D.; Sisney, G.A.; Rademaker, A.W.; Rose, E.; Kaul, K. A single cycle of 2-chlorodeoxyadenosine results in complete remission in the majority of patients with hairy cell leukemia. Blood 1992, 80, 2203–2209. [Google Scholar]
- Robak, T.; Korycka, A.; Kasznicki, M.; Wrzesień-Kuś, A.; Smolewski, P. Purine nucleoside analogues for the treatment of hematological malignancies: Pharmacology and clinical applications. Curr. Cancer Drug Targets 2005, 5, 421–444. [Google Scholar] [CrossRef]
- Johnson, S.A. Nucleoside analogues in the treatment of hematological malignancies. Expert Opin. Pharmacother. 2001, 2, 929–943. [Google Scholar] [CrossRef]
- Robak, T.; Kasznicki, M. Alkylating agents and nucleoside analogues in the treatment of B-cell chronic lymphocytic leukemia. Leukemia 2002, 16, 1015–1027. [Google Scholar] [CrossRef]
- Robak, T.; Lech-Maranda, E.; Korycka, A.; Robak, E. Purine nucleoside analogs as immunosuppressive ans antineoplastic agents: mechanism of action and clinical activity. Curr. Med. Chem. 2006, 13, 3165–3189. [Google Scholar] [CrossRef]
- Pettit, A.R. Mechanism of action of purine analogues in chronic lymphocytic leukemia. Br. J. Haematol. 2003, 121, 692–702. [Google Scholar] [CrossRef]
- Robak, T. Current treatment options in hairy cell leukemia and hairy cell leukemia variant. Cancer Treat. Rev. 2006, 32, 365–376. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Molina-Arcas, M.; Casado, F.J.; Bellosillo, B.; Colomer, D.; Gil, J. Nucleoside transporters in chronic lymphocytic leukemia. Leukemia 2004, 18, 385–393. [Google Scholar] [CrossRef]
- Robak, T. Recent progress in the management of chronic lymphocytic leukemia. Cancer Treat. Rev. 2007, 33, 710–728. [Google Scholar] [CrossRef]
- Kalinka-Warzocha, E.; Wajs, J.; Lech-Maranda, E.; Ceglarek, B.; Holowiecki, J.; Federowicz, I.; Walewski, J.; Czyz, J.; Robak, T.; Warzocha, K. Polish Lymphoma Research Group. Randomized comparison of cladribine alone or in combination with cyclophosphamide, and cyclophosphamide, vincristine and prednisone in previously untreated low-grade B-cell non-Hodgkin lymphoma patients: final report of the Polish Lymphoma Research Group. Cancer 2008, 113, 367–375. [Google Scholar] [CrossRef]
- Zinzani, P.L. Non-Hodgkin's lymphoma: the evolving role of purine analogues. Best Pract. Res. Clin. Haematol. 2002, 15, 505–516. [Google Scholar] [CrossRef]
- Hellmann, A.; Lewandowski, K.; Zaucha, J.M.; Bieniaszewska, M.; Halaburda, K.; Robak, T. Effect of a 2-hour infusion of 2-chlorodeoxyadenosine in the treatment of refractory or previously untreated Waldenström’s macroglobulinemia. Eur. J. Haematol. 1999, 63, 35–41. [Google Scholar]
- Johnson, S.A.; Oscier, D.G.; Leblond, V. Waldenstrom’s macroglobulinaemia. Blood Rev. 2002, 16, 175–184. [Google Scholar] [CrossRef]
- Evens, A.M.; Gartenhaus, R.B. Treatment of T-cell non-Hodgkin’s lymphoma. Curr. Treat Options Oncol. 2004, 5, 289–303. [Google Scholar] [CrossRef]
- Korycka, A.; Lech-Maranda, E.; Robak, T. Novel purine nucleoside analogues for hematological malignancies. Recent Patents AntiCancer Drug Discov. 2008, 3, 123–136. [Google Scholar] [CrossRef]
- Parker, W.B.; Secrist, J.A., 3rd; Waud, W.R. Purine nucleoside antimetabolites in development for the treatment of cancer. Curr. Opin. Investig. Drugs 2004, 5, 592–596. [Google Scholar]
- Faderl, S.; Gandhi, V.; Keating, M.J.; Jeha, S.; Plunkett, W.; Kantarjian, H.M. The role of clofarabine in hematologic and solid malignancies--development of a next-generation nucleoside analog. Cancer 2005, 103, 1985–1995. [Google Scholar] [CrossRef]
- Pui, C.H.; Jeha, S.; Kirkpatrick, P. Clofarabine. Nat. Rev. Drug Discov. 2005, 4, 369–370. [Google Scholar] [CrossRef]
- Roecker, A.M.; Allison, J.C.; Kisor, D.F. Nelarabine: efficacy in the treatment of clinical malignancies. Future Oncol. 2006, 2, 441–448. [Google Scholar] [CrossRef]
- Prus, K.L.; Averett, D.R.; Zimmerman, T.P. Transport and metabolism of 9-beta-d-arabinofuranosylguanine in a human T-lymphoblastoid cell line nitrobenzylthioinosine-sensitive and – insensitive influx. Cancer Res. 1990, 50, 1817–1821. [Google Scholar]
- Kisor, D.F. Nelarabine use in leukemias. Drugs Today 2006, 42, 455–465. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Nimmanapalli, R.; Ravandi, F.; Keating, M.J.; Gandhi, V. Forodesine, an inhibitor of purine nucleoside phosphorylase, induces apoptosis in chronic lymphocytic leukemia cells. Blood 2006, 108, 2392–2398. [Google Scholar] [CrossRef]
- Korycka, A.; Błoński, J.Z.; Robak, T. Forodesine (BCX-1777, Immucillin H)- a new purine nucleoside analogue: mechanism of action and potential clinical application. Mini Rev. Med. Chem. 2007, 7, 976–983. [Google Scholar] [CrossRef]
- Leung, G.; Tse, Ch-M. The role of mitochondrial and plasma mermbrane nucleoside transporters in drug toxicity. Expert Opin Drug Metab Toxicol. 2007, 3, 1–14. [Google Scholar] [CrossRef]
- de Wolf, C.; Jansen, R.; Yamaguchi, H.; de Haas, M.; van de Wetering, K.; Wijnholds, J.; Beijnen, J.; Borst, P. Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther. 2008, 7, 3092–3102. [Google Scholar] [CrossRef]
- Takenaka, K.; Morgan, J.A.; Scheffer, G.L.; Adachi, M.; Stewart, C.F.; Sun, D.; Leggas, M.; Ejendal, K.F.; Hrycyna, C.A.; Schuetz, J.D. Substrate overlap between Mrp4 and Abcg2/Bcrp affects purine analogue drug cytotoxicity and tissue distribution. Cancer Res. 2007, 67, 6965–6972. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Schwab, M.; Takenaka, K.; Nachagari, D.; Morgan, J.; Leslie, M.; Du, W.; Boyd, K.; Cheok, M.; Nakauchi, H.; Marzolini, C.; Kim, R.B.; Poonkuzhali, B.; Schuetz, E.; Evans, W.; Relling, M.; Schuetz, J.D. Transporter-mediated protection against thiopurine-induced hematopoietic toxicity. Cancer Res. 2008, 68, 4983–4989. [Google Scholar] [CrossRef]
- Molina-Arcas, M.; Marce, S.; Villamor, N.; Huber-Ruano, I.; Casado, F.J.; Bellosillo, B.; Montserrat, E.; Gil, J.; Colomer, D. Equilibrative nucleoside transporter-2 (hENT2) protein expression correlates with ex vivo sensitivity to fludarabine in chronic lymphocytic leukemia (CLL) cells. Leukemia 2005, 19, 64–68. [Google Scholar]
- Molina-Arcas, M.; Bellosillo, B.; Casado, F.J.; Montserrat, E.; Gil, J.; Colomer, D.; Pastor-Anglada, M. Fludarabine uptake mechanisms in B-cell chronic lymphocytic leukemia. Blood 2003, 101, 2328–2334. [Google Scholar] [CrossRef]
- Mackey, J.R.; Galmarini, C.M.; Graham, K.A.; Joy, A.A.; Delmar, A.; Dabbagh, L.; Glubrecht, D.; Jewell, L.D.; Lai, R.; Lang, T.; Hanson, J.; Young, J.D.; Merle-Beral, H.; Binet, J.L.; Cass, C.E.; Dumontet, C. Quantitative analysis of nucleoside transporter and metabolism gene expression in chronic lymphocytic leukemia (CLL): identification of fludarabine-sensitive and -insensitive populations. Blood 2005, 105, 767–774. [Google Scholar] [CrossRef]
- Rodriquez, C.O.; Mitchell, B.S.; Ayres, M.; Eriksson, S.; Gandhi, V. Arabinosylguanine is phosphorylated by both cytoplasmic deoxycytidine kinase and mitochondrial deoxyguanosine kinase. Cancer Res. 2002, 62, 3100–3105. [Google Scholar]
- Van den Neste, E.; Cardoen, S.; Offner, F.; Bontemps, F. Old and new insights into the mechanisms of action of two nucleoside analogs active in lymphoid malignancies: fludarabine and cladribine (review). Intern. J. Oncol. 2005, 27, 1113–1124. [Google Scholar]
- Adkins, J.C.; Peters, D.H.; Markham, A. Fludarabine. An update of its pharmacology and use in the treatment of haematological malignancies. Drugs 1997, 53, 1005–1037. [Google Scholar]
- Malspeis, L.; Grever, M.R.; Staubus, A.E.; Young, D. Pharmacokinetics of 2-F-ara-A (9-beta-d-arabinofuranosyl-2-fluoroadenine) in cancer patients during the phase I clinical investigation of fludarabine phosphate. Semin. Oncol. 1990, 17, 18–32. [Google Scholar]
- Hutton, J.J.; Von Hoff, D.D.; Kuhn, J.; Phillips, J.; Hersh, M.; Clark, G. Phase I clinical investigation of 9-beta-d-arabinofuranosyl-2-fluoroadenine 5'-monophosphate (NSC 312887), a new purine antimetabolite. Cancer Res. 1984, 44, 4183–4186. [Google Scholar]
- Foran, J.M.; Oscier, D.; Orchard, J.; Johnson, S.A.; Tighe, M.; Cullen, M.H.; de Takats, P.G.; Kraus, C.; Klein, M.; Lister, TA. Pharmacokinetic study of single doses of oral fludarabine phosphate in patients with "low-grade" non-Hodgkin's lymphoma and B-cell chronic lymphocytic leukemia. J. Clin. Oncol. 1999, 17, 1574–1579. [Google Scholar]
- Boogaerts, M.A. Oral fludarabine therapy in chronic lymphocytic leukemia--increased convenience. Hematology J. 2004, 5, 31–37. [Google Scholar] [CrossRef]
- Beutler, E. Cladribine (2-chlorodeoxyadenosine). Lancet 1992, 340, 952–956. [Google Scholar] [CrossRef]
- Carson, D.A.; Wasson, D.B.; Taetle, R.; Yu, A. Specific toxicity of 2-chlorodeoxyadenosine toward resting and proliferating human lymphocytes. Blood 1983, 62, 737–743. [Google Scholar]
- Seto, S.; Carrera, C.J.; Kubota, M.; Wasson, D.B.; Carson, D.A. Mechanism of deoxyadenosine and 2-chlorodeoxyadenosine toxicity to nondividing human lymphocytes. J. Clin. Invest. 1985, 75, 377–383. [Google Scholar]
- Griffig, J.; Koob, R.; Blakley, R.L. Mechanism of inhibition of DNA synthesis by 2-chlorodeoxyadenosine in human lymphoblastic cells. Cancer Res. 1989, 49, 6923–6928. [Google Scholar]
- Robertson, L.E.; Chubb, S.; Meyn, R.E.; Story, M.; Ford, R.; Hittelman, W.N.; Plunkett, W. Induction of apoptotic cell death in chronic lymphocytic leukemia 2-chloro-2’-deoxyadenosine and 9-β-d-arabinosyl-2-fluoroadenine. Blood 1993, 81, 143–150. [Google Scholar]
- Carson, D.A.; Carrera, C.J.; Wasson, D.B.; Yamanaka, H. Proogrammed cell death and adenine deoxynucleotide metabolism in humans lymphocytes. Adv. Enzyme Regul. 1988, 27, 395–404. [Google Scholar] [CrossRef]
- Kawasaki, H.; Carrera, C.J.; Piro, L.D.; Saven, A.; Kipps, T.J.; Carson, D.A. Relationship of deoxycytydine kinase and cytoplasmic 5’nucloetidase to the chemothrapeutic efficacy of 2-chlorodeoxyadenosine. Blood 1993, 81, 597–601. [Google Scholar]
- Marzo, I.; Perez-Gala, P.; Rubio-Felix, D.; Anel, A.; Naval, J. Cladribine induces apoptosis in human leukemia cells by caspase-dependent and –independent pathways acting on mitochondra. Biochemistry 2001, 359, 537–546. [Google Scholar] [CrossRef]
- Nomura, Y.; Inanami, O.; Takahashi, K.; Matsuda, A.; Kuwabara, M. 2-Chloro-2’-deoxyadenosine induces apoptosis through the Fas/Fas ligand pathway in human leukemia cell line MOLT-4. Leukemia 2000, 14, 299–306. [Google Scholar] [CrossRef]
- Johnston, J.B.; Daeninck, P.; Verburg, L.; Lee, K.; Williams, G.; Israels, L.G.; Mowat, M.R.; Begleiter, A. P53, mdm-2, bax and bcl-2 and drug resistance in chronic lymphocytic leukemia. Leuk. Lymphoma 1997, 26, 425–449. [Google Scholar]
- Genini, D.; Budihardjo, I.; Plunkett, W.; Wang, X.; Carrera, C.J.; Cottam, H.B.; Carson, D.A.; Leoni, L.M. Nucleotide requirements for the in vitro activation of the apoptosis protein-activating factor-1-mediated caspase pathway. J. Biol. Chem. 2000, 275, 29–34. [Google Scholar] [CrossRef]
- Grutter, M.G. Caspases: key players in programmed cell death. Curr. Opinion Struct. Biol. 2000, 10, 649–655. [Google Scholar] [CrossRef]
- Bosanquet, A.G.; Sturm, I.; Wieder, T.; Essmann, F.; Bosanquet, M.I.; Head, D.J.; Dörken, B.; Daniel, P.T. Bax expression correlates with cellular drug sensitivity to doxorubicin, cyclophosphamide and chlorambucil but not fludarabine, cladribine or corticosteroids in B-cell chronic lymphocytic leukemia. Leukemia 2002, 16, 1035–1044. [Google Scholar] [CrossRef]
- Klopfer, A.; Hasenjager, A.; Belka, C.; Schulze-Osthoff, K.; Dorken, B.; Daniel, P. Adenine deoxynucleotides fludarabine and cladribine induces apoptosis in a CD95/Fas receptor, FADD and caspase-8- independent manner by activation of the mitochondrial cell death pathway. Oncogene 2004, 16, 9408–9418. [Google Scholar]
- Johnson, S.A. Clinical pharmacokinetics of nucleoside analogues. Focus on haematological malignancies. Clin. Pharm. 2000, 39, 5–26. [Google Scholar] [CrossRef]
- Saven, A.; Cheung, W.K.; Smith, I.; Moyer, M.; Johannsen, T.; Rose, E.; Gollard, R.; Kosty, M.; Miller, W.E.; Piro, L.D. Pharmacokinetics study of oral and bolus intravenous 2-chlorodeoxyadenosine in patients with malignancy. J. Clin. Oncol. 1996, 14, 978–983. [Google Scholar]
- Liliemark, J.; Albertioni, F.; Hassan, M.; Juliusson, G. One the bioavailability of oral and subcutaneous 2-chloro-2’-deoxyadenosine in humans: alternative routes of administration. J. Clin. Oncol. 1992, 10, 1514–1518. [Google Scholar]
- Robak, T.; Błasińska-Morawiec, M.; Krykowski, E.; Hansz, J.; Komarnicki, M.; Kazimierczak, M.; Konopka, L.; Maj, S.; Hellmann, A.; Zaucha, J.M.; Urasiński, L.; Zdziarska, B.; Kotlarek-Haus, S.; Usnarska-Zubkiewicz, L.; Kuratowska, Z.; Dwilewicz-Trojaczek, J.; Hołowiecki, J.; Krawczyk-Kulis, M.; Grieb, P. 2-chlorodeoxyadenosine (2-CdA) in 2-hour versus 24-hour intravenous infusion in the treatment of patients with hairy cell leukemia. Leuk. Lymphoma 1996, 22, 107–111. [Google Scholar] [CrossRef]
- Liliemark, J.; Juliusson, G. On the pharmacokinetics of 2-chloro-2’-deoxyadenosine in humans. Cancer Res. 1991, 51, 5570–5572. [Google Scholar]
- Albertioni, F.; Pettersson, B.; Reichelová, V.; Juliusson, G.; Liliemark, J. Analysis of 2-chloro-2’-deoxyadenosine in human blood plasma and urine by high-performance liquid chromatography using solid-phase extraction. The Drug Monit 1994, 16, 413–418. [Google Scholar] [CrossRef]
- Liliemark, J.; Pettersson, B.; Juliusson, G. Determination of 2-chloro-2’-deoxyadenosine in human plasma. Biomed. Chromatogr. 1991, 5, 262–264. [Google Scholar] [CrossRef]
- Lindemalm, S.; Savic, R.M.; Karlsson, M.O.; Liliemark, J.; Juliusson, G.; Albertioni, F. Application of population pharmacokinetics to cladribine. BMC Pharmacol. 2005, 9,5, 4. [Google Scholar]
- Robak, T.; Korycka, A.; Robak, E. Older and new formulations of cladribine. Pharmacology and clinical efficacy in hematological malignancies. Recent Patents Anticancer Drug Discov. 2006, 1, 23–38. [Google Scholar] [CrossRef]
- Juliusson, G.; Heldal, D.; Hippe, E.; Hedenus, M.; Malm, C.; Wallman, K.; Stolt, C.M.; Evensen, S.A.; Albertioni, F.; Tjønnfjord, G. Subcutaneous injections of 2-Chlorodeoxyadenosine for symptomatic hairy cell leukemia. J. Clin. Oncol. 1995, 13, 989–995. [Google Scholar]
- Aldinucci, D.; Poletto, D.; Lorenzon, D.; Nanni, P.; Degan, K.O.; Rapana, B.; Pinto, A.; Gattei, V. CD26 expression correlates with a reduced sensitivity to 2'-deoxycoformycin-induced growth inhibition and apoptosis in T-cell leukemia/lymphomas. Clin. Cancer Res. 2004, 10, 508–520. [Google Scholar] [CrossRef]
- Brogden, R.N.; Sorkin, E.M. Pentostatin: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in lymphoproliferative disorders. Drugs 1993, 4, 652–677. [Google Scholar] [CrossRef]
- Niitsu, N.; Yamaguchi, Y.; Umeda, M.; Honma, Y. Human monocytoid leukemia cells are highly sensitive to apoptosis induced by 2'-deoxycoformycin and 2'-deoxyadenosine: association with dATP-dependent activation of caspase-3. Blood 1998, 92, 3368–3375. [Google Scholar]
- Venner, P.M.; Glazer, R.I.; Blatt, J.; Sallan, S.; Rivera, G.; Holcenberg, J.S.; Lipton, J.; Murphy, S.B.; Poplack, D.G. Levels of 2'-deoxycoformycin, adenosine, and deoxyadenosine in patients with acute lymphoblastic leukemia. Cancer Res. 1981, 41, 4508–4511. [Google Scholar]
- Staubus, A.E.; Weinrib, A.B.; Malspeis, L. An enzymatic kinetic method for the determination of 2'-deoxycoformycin in biological fluids. Biochem. Pharmacol. 1984, 33, 1633–1637. [Google Scholar] [CrossRef]
- Smyth, J.F.; Paine, R.M.; Jackman, A.L.; Harrap, K.R.; Chassin, M.M.; Adamson, R.H.; Johns, D.G. The clinical pharmacology of the adenosine deaminase inhibitor 2'-deoxycoformycin. Cancer. Chemother. Pharmacol. 1980, 5, 93–101. [Google Scholar] [CrossRef]
- Major, P.P.; Agarwal, R.P.; Kufe, D.W. Clinical pharmacology of deoxycoformycin. Blood 1981, 58, 91–96. [Google Scholar]
- Grever, M.R.; Siaw, M.F.; Jacob, W.F.; Neidhart, J.A.; Miser, J.S.; Coleman, M.S.; Hutton, J.J.; Balcerzak, S.P. The biochemical and clinical consequences of 2'-deoxycoformycin in refractory lymphoproliferative malignancy. Blood 1981, 57, 406–417. [Google Scholar]
- Pui, C.H.; Jeha, S.; Kirkpatrick, P. Clofarabine. Nat. Rev. Drug Discov. 2005, 4, 369–370. [Google Scholar] [CrossRef]
- Lindemalm, S.; Liliemark, J.; Juliusson, G.; Larsson, R.; Albertioni, F. Cytotoxicity and pharmacokinetics of cladribine metabolite, 2-chloroadenine in patients with leukemia. Cancer Lett. 2004, 210, 171–177. [Google Scholar] [CrossRef]
- Bonate, P.L.; Arthaud, L.; Cantrell, W.R.Jr.; Stephenson, K.; Secrist, J.A., 3rd; Weitman, S. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 855–863. [Google Scholar] [CrossRef]
- Reichelova, V.; Liliemark, J.; Albertioni, F. Liquid chromatographic study of acid stability of 2-chloro-2'-arabino-fluoro-2'-deoxyadenosine, 2-chloro-2'-deoxy-adenosine and related analogues. J. Pharm. Biomed. Anal. 1995, 13, 711–714. [Google Scholar] [CrossRef]
- King, K.M.; Damaraju, V.L.; Vickers, M.F.; Yao, S.Y.; Lang, T.; Tackaberry, T.E.; Mowles, D.A.; Ng, A.M.; Young, J.D.; Cass, C.E. A comparison of the transportability, and its role in cytotoxicity, of clofarabine, cladribine, and fludarabine by recombinant human nucleoside transporters produced in three model expression systems. Mol. Pharmacol. 2006, 69, 346–353. [Google Scholar]
- Lotfi, K.; Månsson, E.; Spasokoukotskaja, T.; Pettersson, B.; Liliemark, J.; Peterson, C.; Eriksson, S.; Albertioni, F. Biochemical pharmacology and resistance to 2-chloro-2'-arabino-fluoro-2'-deoxyadenosine, a novel analogue of cladribine in human leukemic cells. Clin. Cancer Res. 1999, 5, 2438–2444. [Google Scholar]
- Zhang, Y.; Secrist, J.A., 3rd; Ealick, S.E. The structure of human deoxycytidine kinase in complex with clofarabine reveals key interactions for prodrug activation. Acta Crystallogr. D.Biol. Crystallogr. 2006, 62, 133–139. [Google Scholar]
- Parker, W.B.; Shaddix, S.C.; Rose, L.M.; Shewach, D.S.; Hertel, L.W.; Secrist, J.A., 3rd; Montgomery, J.A.; Bennett, L.L., Jr. Comparison of the mechanism of cytotoxicity of 2-chloro-9-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)adenine, 2-chloro-9-(2-deoxy-2-fluoro-beta-d-ribofuranosyl)adenine, and 2-chloro-9-(2-deoxy-2,2-difluoro-beta-d-ribofuranosyl)adenine in CEM cells. Mol. Pharmacol. 1999, 55, 515–520. [Google Scholar]
- Gandhi, V.; Kantarjian, H.; Faderl, S.; Bonate, P.; Du, M.; Ayres, M.; Rios, M.B.; Keating, M.J.; Plunkett, W. Pharmacokinetics and pharmacodynamics of plasma clofarabine and cellular clofarabine triphosphate in patients with acute leukemias. Clin. Cancer Res. 2003, 9, 6335–6342. [Google Scholar]
- Xie, K.C.; Plunkett, W. Deoxynucleotide pool depletion and sustained inhibition of ribunucleotide reductase and DNA synthesis after treatment of human lymphoblastoid cells with 2-chloro-9-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl) adenine. Cancer Res. 1996, 56, 3030–3037. [Google Scholar]
- Genini, D.; Adachi, S.; Chao, Q.; Rose, D.W.; Carrera, C.J.; Cottam, H.B.; Carson, D.A.; Leoni, L.M. Deoxyadenosine analogs induce programmed cell death in chronic lymphocytic leukemia cells by damaging the DNA and by directly affecting the mitochondria. Blood 2000, 96, 3537–3543. [Google Scholar]
- Jeha, S.; Gandhi, V.; Chan, K.W.; McDonald, L.; Ramirez, I.; Madden, R.; Rytting, M.; Brandt, M.; Keating, M.; Plunkett, W.; Kantarjian, H. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood 2004, 103, 784–789. [Google Scholar]
- Kantarjian, H.; Gandhi, V.; Cortes, J.; Verstovsek, S.; Du, M.; Garcia-Manero, G.; Giles, F.; Faderl, S.; O'Brien, S.; Jeha, S.; Davis, J.; Shaked, Z.; Craig, A.; Keating, M.; Plunkett, W.; Freireich, E.J. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood 2003, 102, 2379–2386. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Gandhi, V.; Kozuch, P.; Faderl, S.; Giles, F.; Cortes, J.; O'Brien, S.; Ibrahim, N.; Khuri, F.; Du, M.; Rios, M.B.; Jeha, S.; McLaughlin, P.; Plunkett, W.; Keating, M. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J. Clin. Oncol. 2003, 21, 1167–1173. [Google Scholar]
- Roecker, A.M.; Allison, J.C.; Kisor, D.F. Nelarabine: efficacy in the treatment of clinical malignancies. Future Oncol. 2006, 2, 441–448. [Google Scholar] [CrossRef]
- Prus, K.L.; Averett, D.R.; Zimmerman, T.P. Transport and metabolism of 9-beta-d-arabinofuranosylguanine in a human T-lymphoblastoid cell line nitrobenzylthioinosine-sensitive and –insensitive influx. Cancer Res. 1990, 50, 1817–1821. [Google Scholar]
- Rodriguez, C.O.J.; Mitchell, B.S.; Ayres, M.; Eriksson, S.; Gandhi, V. Arabinosylguanine is phosphorylated by both cytoplasmic deoxycytidine kinase and mitochondrial deoxyguanosine kinase. Cancer Res. 2002, 62, 3100–3105. [Google Scholar]
- Rodriguez, C.O.Jr.; Stellrecht, C.M.; Gandhi, V. Mechanisms for T-cell selective cytotoxicity of arabinosylguanine. Blood 2003, 102, 1842–1848. [Google Scholar] [CrossRef]
- Kline, J.P.; Larson, R. Clofarabine in the treatment of acute myeloid leukemia and acute lymphoblastic leukemia: a review. Expert Opin. Pharmacother. 2005, 6, 1–8. [Google Scholar] [CrossRef]
- Ghandi, V.; Plunkett, W. Clofarabine and nelarabine: two new purine nucleoside analogs. Curr. Opini. Oncol. 2006, 18, 584–590. [Google Scholar] [CrossRef]
- Beesley, A.H.; Palmer, M.L.; Ford, J.; Weller, R.E.; Cummings, A.J.; Freitas, J.R.; Firth, M.J.; Perera, K.U.; de Klerk, N,H,.; Kees, U.R. Beesley, A.H.; Palmer, M.L.; Ford ,J.; Weller, R.E.; Cummings, A.J.; Freitas, J.R.; Firth, M.J.; Perera, K.U.; de Klerk, N,H,.; Kees, U.R. Br. J. Haematol. 2007, 137, 109–116. [Google Scholar]
- Kisor, D.F.; Plunkett, W.; Kurtzberg, J.; Mitchell, B.; Hodge, J.P.; Ernst, T.; Keating, M.J.; Gandhi, V. Pharmacokinetics of nelarabine and 9-beta-d-arabinofuranosyl guanine in pediatric and adult patients during a phase I study of nelarabine for the treatment of refractory hematologic malignancies. J. Clin. Oncol. 2000, 18, 995–1003. [Google Scholar]
- Gandhi, V.; Plunkett, W.; Rodriguez, C.O., Jr.; Nowak, B.J.; Du, M.; Ayres, M.; Kisor, D.F.; Mitchell, B.S.; Kurtzberg, J.; Keating, M.J. Compound GW506U78 in refractory hematologic malignancies: relationship between cellular pharmacokinetics and clinical response. J. Clin. Oncol. 1998, 16, 3607–3615. [Google Scholar]
- Evans, G.B.; Furneaux, R.H.; Lewandowicz, A.; Schramm, V.L.; Tyler, P.C. Synthesis of second-generation transition state analogues of human purine nucleoside phosphorylase. J. Med. Chem. 2003, 46, 5271–5276. [Google Scholar] [CrossRef]
- Clinch, K.; Evans, G.B.; Fleet, G.W.; Furneaux, R.H.; Johnson, S.W.; Lenz, D.H.; Mee, S.P.; Rands, P.R.; Schramm, V.L.; Taylor Ringia, E.A.; Tyler, P.C. Synthesis and bio-activities of L-enantiomers of two potent transition state analogue inhibitor of purine nucleoside phosphorylases. Org. Biomol. Chem. 2006, 4, 1131–1139. [Google Scholar] [CrossRef]
- Canduri, F.; Silva, R.G.; dos Santos, D.M.; Palma, M.S.; Basso, L.A.; Santos, D.S.; de Azevedo, W.F., Jr. Structure of human PNP complexed with ligands. Acta Crystallogr D. Biol. Crystallogr. 2005, 61, 856–862. [Google Scholar] [CrossRef]
- Bantia, S.; Miller, P.J.; Parker, C.D.; Ananth, S.L.; Horn, L.H.; Kilpatrick, J.M.; Morris, P.E.; Hutchison, T.L.; Montgomery, J.A.; Sandhu, J.S. Purine nucleoside phosphorylase inhibitor BCX-1777 (Immucillin-H)- a novel potent and orally active immunosuppressive agent. Intern. Immunopharmacol. 2001, 1, 1199–1210. [Google Scholar] [CrossRef]
- Kicska, G.A.; Long, L.; Horig, H.; Fatrchild, C.; Tyler, P.C.; Furneaux, R.H.; Schramm, V.L.; Kaufman, H.L. Immucillin H, a powerful transition-state analog inhibitor of purine nucleoside phosphorylase, selectively inhibits human T lymphocytes. PNAS 2001, 98, 4593–4598. [Google Scholar] [CrossRef]
- Banthia, S.; Kilpatrick, J.M. Purine nucleoside phosphorylase inhibitors in T-cell malignancies. Curr. Oppinion Drug Discov. Develop. 2004, 7, 243–247. [Google Scholar]
- Ashcroft, M.; Kubbutat, M.H.; Vousden, K.H. Regulation of p53 function and stability by phosphorylation. Mol.Cell Biol. 1999, 19, 1751–1758. [Google Scholar]
- Kilpatrick, J.M.; Morris, P.E.; Serota, D.G., jr.; Phillips, D.; Moore, D.R.; Bennett, J.C.; Babu, Y.S. Intravenous and oral pharmacokinetic study of BCX-1777, a novel purine nucleoside phosphorylase transition-state inhibitor. In vivo effects on blood 2'-deoxyguanosine in primates. Int. Immunopharmacol. 2003, 3, 541–548. [Google Scholar] [CrossRef]
- Piro, L.D.; Miller, W.E.; Carrera, C.J.; Carson, D.A.; Beutler, E. Hairy cell leukemia. N. Engl. J. Med. 1987, 317, 901–902, (letter). [Google Scholar] [CrossRef]
- Cheson, B.D.; Sorensen, J.M.; Vena, D.A. Treatment of hairy cell leukemia with 2-chlorodeoxyadenosine via the Group C Protocol mechanism of the National Cancer Institute: a report of 979 patients. J. Clin. Oncol. 1998, 16, 3007–3015. [Google Scholar]
- Juliusson, G.; Lenkei, R.; Liliemark, J. Flow cytometry of blood and bone marrow cells from patients with hairy cell leukemia: phenotype of hairy cell and lymphocyte subsets after treatment with 2-chlorodeoxyadenosie. Blood 1994, 83, 3672–3681. [Google Scholar]
- von Rohr, A.; Schmitz, S.F.; Tichelli, A.; Hess, U.; Piguet, D.; Wernli, M.; Frickhofen, N.; Konwalinka, G.; Zulian, G.; Ghielmini, M.; Rufener, B.; Racine, C.; Fey, M.F.; Cerny, T.; Betticher, D.; Tobler, A. Swiss Group for Clinical Cancer Research (SAKK), Bern, Switzerland. Treatment of hairy cell leukemia with cladribine (2-chlorodeoxyadenosine) by subcutaneous bolus injection: a phase II study. Ann. Oncol. 2002, 13, 1641–1649. [Google Scholar] [CrossRef]
- Tallman, M.S.; Zakarija, A. Hairy cell leukemia survival and relapse: Long-term follow-up of purine analogs-based therapy and approach for relapsed disease. Transfus Apheresis Sci. 2005, 32, 90–103. [Google Scholar]
- Saven, A.; Burian, C.; Kozioł, J.A.; Piro, L.D. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood 1998, 92, 1918–1926. [Google Scholar]
- Bastie, J.N.; Cazals-Hatem, D.; Daniel, M.T.; D'Agay, M.F.; Rabian, C.; Glaisner, S.; Noel-Walter, M.P.; Dabout, D.; Flandrin, G.; Dombret, H.; Poisson, D.; Degos, L.; Castaigne, S. Five years follow-up after 2-chlorodeoxyadenosine treatment in thirty patients with hairy cell leukemia: evaluation of minimal residual disease and CD4+ lymphocytopenia after treatment. Leuk. Lymphoma 1999, 35, 555–565. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Tani, M.; Marchi, E.; Stefoni, V.; Alinari, L.; Musuraca, G.; Gabriele, A.; Pileri, S.; Baccarani, M. Long-term follow-up of front line treatment of hairy cell leukemia with 2-chlorodeoxyadenosine. Haematol. 2004, 89, 309–313. [Google Scholar]
- Goodman, G.R.; Burian, C.; Koziol, J.A.; Saven, A. Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. J. Clin. Oncol. 2003, 21, 891–896. [Google Scholar] [CrossRef]
- Hoffman, M.A.; Janson, D.; Rose, E.; Rai, K.R. Treatment of hairy cell leukemia with cladribine: response , toxicity and long-term follow-up. J. Clin. Oncol. 1997, 15, 1138–1142. [Google Scholar]
- Robak, T.; Błasińska-Morawiec, M.; Błoński, J.; Hellmann, A.; Hałaburda, K.; Konopka, L.; Kotlarek-Haus, S.; Potoczek, S.; Hansz, J.; Dmoszyńska, A.; Urasiński, I.; Zdziarska, B.; Dwilewicz-Trojaczek, J.; Hołowiecki, J.; Skotnicki, A.B. 2-chlorodeoxyadenosine: (cladribine) in the treatment of hairy cell leukemia and hairy cell leukemia variant 7-year experience in Poland. Eur. J. Haematol. 1999, 62, 49–56. [Google Scholar]
- Lauria, F.; Boachia, M.; Marotta, G.; Respadori, D.; Zinzani, P.L.; Rondelli, D. Weekly administration of 2-chlorodeoxyadenosine in patients with hairy cell leukemia is effective and reduces infectious complications. Haematol. 1999, 84, 22–25. [Google Scholar]
- Robak, T.; Jamroziak, K.; Gora-Tybor, J.; Blonski, J.Z.; Kasznicki, M.; Dwilewicz-Trojaczek, J.; Wiater, E.; Zdunczyk, A.; Dybowicz, J.; Dmoszynska, A.; Wojtaszko, M.; Zdziarska, B.; Calbecka, M.; Kostyra, A.; Hellmann, A.; Lewandowski, K.; Stella-Holowiecka, B.; Sulek, K.; Gawronski, K.; Skotnicki, A.B.; Nowak, W.; Zawilska, K.; Molendowicz-Portala, L.; Kloczko, J.; Sokolowski, J.; Warzocha, K.; Seferynska, I.; Ceglarek, B.; Konopka, L. Cladribine in a weekly versus daily schedule for untreated active hairy cell leukemia: final report from the Polish Adult Leukemia Group (PALG) of a prospective randomized, multicenter trial. Blood 2007, 109, 3672–3675. [Google Scholar] [CrossRef]
- Liliemark, J.; Albertioni, F.; Hassan, M.; Juliusson, G. On the bioavailability of oral and subcutaneous 2-chloro-2-deoxyadenosine in humans: alternative routes of administration. J. Clin. Oncol. 1992, 10, 1514–1518. [Google Scholar]
- Juliusson, G.; Heldal, D.; Hippe, E.; Hedenus, M.; Malm, C.; Wallman, K.; Stolt, C.M.; Evensen, S.A.; Albertioni, F.; Tjønnfjord, G.; et al. Subcutaneous injections of 2-chlorodeoxyadenosine for symptomatic hairy cell leukemia. J. Clin. Oncol. 1995, 13, 989–995. [Google Scholar]
- Spiers, A.S.; Parekh, S.J.; Bishop, M.B. Hairy cell leukemia: induction of complete remission with pentostatin (2’-deoxycoformycin). J. Clin. Oncol. 1984, 2, 1336–1342. [Google Scholar]
- Spiers, A.S.; Moore, D.; Cassileth, P.A.; Harrington, D.P.; Cummings, F.J.; Neiman, R.S.; Bennett, J.M; O'Connell, M.J. Remissions in hairy cell leukemia with pentostatin (2’-deoxycoformycin). N. Engl. J. Med. 1987, 316, 825–830. [Google Scholar] [CrossRef]
- Kraut, E.H.; Bouroncle, B.A.; Grever, M.R. Pentostatin in the treatment of advanced hairy cell leukemia. J. Clin. Oncol. 1989, 7, 168–172. [Google Scholar]
- Grever, M.R.; Doan, C.A.; Kraut, E.H. Pentostatin in the treatment of hairy cell leukemia. Best Pract. Res. Clin. Haematol. 2003, 16, 91–99. [Google Scholar] [CrossRef]
- Thaler, J.; Grünewald, K.; Gattringer, C.; Ho, A.D.; Weyrer, K.; Dietze, O.; Stauder, R.; Fluckinger, T.; Lang, A.; Huber, H. Long-term follow-up of patients with hairy cel leukaemia treated with pentostatin: lymphocyte subpopulations and residual bone marrow infiltration. Br. J. Haematol. 1993, 84, 75–82. [Google Scholar] [CrossRef]
- Dearden, C.R.; Matutes, E.; Hilditch, B.T.; Wansbury, G.J.; Catovsky, D. Long term follow-up patients with hairy cell leukemia after treatment with pentostatin or cladribine. Br. J. Haematol. 1999, 106, 515–519. [Google Scholar] [CrossRef]
- Ribeiro, P.; Bouaffia, F.; Peaud, P.Y.; Blanc, M.; Salles, B.; Salles, G.; Coiffier, B. Long-term outcome of patients with hairy cell leukemia treated with pentostatin. Cancer 1999, 85, 65–71. [Google Scholar] [CrossRef]
- Rafel, M.; Cervantes, F.; Beltrán, J.M.; Zuazu, F.; Hernández Nieto, L.; Rayón, C.; García Talavera, J.; Montserrat, E. Deoxycoformycin in the treatment of patients with hairy cell leukemia: results of a Spanish collaborative study of 80 patients. Cancer 2000, 88, 352–357. [Google Scholar] [CrossRef]
- Johnston, J.B.; Eisenhauer, E.; Wainman, N.; Corbett, W.E.; Zaentz, S.D.; Daeninck, P.J. Long-term outcome following treatment of hairy cell leukemia with pentostatin (Nipent): a National Cancer Institute of Canada study. Semin. Oncol. 2000, 27, 32–36. [Google Scholar]
- Habermann, T.M.; Andersen, J.W.; Cassileth, P.A.; Bennett, J.M.; Oken, M.M. Sequential administration of recombinant interferon alpha and deoxycoformycin in the treatment of hairy cell leukaemia. Br. J. Haematol. 1992, 80, 466–471. [Google Scholar] [CrossRef]
- Flinn, I.W.; Kopecky, K.J.; Foucar, M.K.; Head, D.; Bennett, J.M.; Hutchison, R.; Corbett, W.; Cassileth, P.; Habermann, T.; Golomb, H.; Rai, K.; Eisenhauer, E.; Appelbaum, F.; Cheson, B.; Grever, M.R. Long-term follow-up of remission duration, mortality and second malignancies in hairy cell leukemia patients treated with pentostatin. Blood 2000, 96, 2981–2986. [Google Scholar]
- Maloisel, F.; Benboubker, L.; Gardembas, M.; Coiffier, B.; Divine, M.; Sebban, C.; Blanc, M.; Abgrall, J.F.; Lederlin, P.; Harousseau, J.L.; Blaise, A.M.; Grosbois, B.; Morice, P.; Ghandour, C.; Castaigne, S. Long-term outcome with pentostatin treatment in hairy cell leukemia patients. A French retrospective study of 238 patients. Leukemia 2003, 17, 45–51. [Google Scholar] [CrossRef]
- Grever, M.; Kopecky, K.; Foucar, M.K.; Head, D.; Bennett, J.M.; Hutchison, R.E.; Corbett, W.E.; Cassileth, P.A.; Habermann, T.; Golomb, H.; et al. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: an Intergroup study. J. Clin. Oncol. 1995, 13, 974–982. [Google Scholar]
- Else, M.; Ruchlemer, R.; Osuji, N.; Del Giudice, I.; Matutes, E.; Woodman, A.; Wotherspoon, A.; Swansbury, J.; Dearden, C.; Catovsky, D. Long remissions in hairy cell leukemia with purine analogs. A report of 219 patients with a median follow-up of 12.5 years. Cancer 2005, 104, 2442–2448. [Google Scholar] [CrossRef]
- Kantarjian, H.; Schachner, J.; Keating, M.J. Fludarabine therapy in hairy cell leukemia. Cancer 1991, 67, 1291–1293. [Google Scholar] [CrossRef]
- Rai, K.R.; Peterson, B.L.; Appelbaum, F.R.; Kolitz, J.; Elias, L.; Shepherd, L.; Hines, J.; Threatte, G.A.; Larson, R.A.; Cheson, B.D.; Schiffer, C.A. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N. Engl. J. Med. 2000, 343, 1760–1767. [Google Scholar]
- Robak, T.; Bloński, J.Z.; Kasznicki, M.; Blasińska-Morawiec, M.; Krykowski, E.; Dmoszyńska, A.; Mrugala-Spiewak, H.; Skotnicki, A.B.; Nowak, W.; Konopka, L.; Ceglarek, B.; Maj, S.; Dwilewicz-Trojaczek, J.; Hellmann, A.; Urasiński, I.; Zdziarska, B.; Kotlarek-Haus, S.; Potoczek, S.; Grieb, P. Cladribine with prednisone versus chlorambucil with prednisone as first-line therapy in chronic lymphocytic leukemia: report of a prospective, randomized, multicenter trial. Blood 2000, 96, 2723–2729. [Google Scholar]
- Robak, T.; Błoński, J.Z.; Kasznicki, M.; Góra-Tybor, J.; Dmoszyńska, A.; Wojtaszko, M.; Skotnicki, A.B.; Nowak, W.; Hellmann, A.; Lewandowski, K.; Zdziarska, B.; Konopka, L.; Ceglarek, B.; Dwilewicz-Trojaczek, J.; Boguradzki, P.; Kuliczkowski, K.; Sułek, K.; Warzocha, K. Comparison of cladribine plus prednisone with chlorambucil plus prednisone in patients with chronic lymphocytic leukemia. Final report of the Polish Adult Leukemia. Final report of the Polish Adult Leukemia Group (PALG CLL1). Med. Sci. Monit. 2005, 11, 171–180. [Google Scholar]
- Johnson, S.; Smith, A.G.; Löffler, H.; Osby, E.; Juliusson, G.; Emmerich, B.; Wyld, P.J.; Hiddemann, W.; The French Cooperative Group on CLL. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin and prednisone (CAP) for treatment of advanced stage chronic lymphocytic leukemia. Lancet 1996, 347, 1432–1438. [Google Scholar]
- Leporrier, M.; Chevret, S.; Cazin, B.; Boudjerra, N.; Feugier, P.; Desablens, B.; Rapp, M.J.; Jaubert, J.; Autrand, C.; Divine, M.; Dreyfus, B.; Maloum, K.; Travade, P.; Dighiero, G.; Binet, J.L.; Chastang, C.; The French Cooperative Group on Chronic Lymphocytic Leukemia. Randomized comparison of fludarabine, CAP, and CHOP in 938 previously untreated stage B and C chronic lymphocytic leukemia patients. Blood 2001, 98, 2319–2325. [Google Scholar] [CrossRef]
- Keating, M.J.; O'Brien, S.; Kantarjian, H.; Plunkett, W.; Estey, E.; Koller, C.; Beran, M.; Freireich, E.J. Long-term follow-up of patients with chronic lymphocytic leukemia treated with fludarabine as a single agent. Blood 1993, 81, 2878–2884. [Google Scholar]
- Steurer, M.; Pall, G.; Richards, S.; Bohlius, J.; Greil, R.; Cochrane Haematologic Malignancies Group. Single agent purine analogues for the treatment of chronic lymphocytic leukemia: a systematic review and meta- analysis. Cancer Treat. Rev. 2006, 32, 377–389. [Google Scholar] [CrossRef]
- Robak, T.; Bloński, J.Z.; Kasznicki, M.; Konopka, L.; Ceglarek, B.; Dmoszyńska, A.; Soroka-Wojtaszko, M.; Skotnicki, A.B.; Nowak, W.; Dwilewicz-Trojaczek, J.; Tomaszewska, A.; Hellmann, A.; Lewandowski, K.; Kuliczkowski, K.; Potoczek, S.; Zdziarska, B.; Hansz, J.; Kroll, R.; Komarnicki, M.; Holowiecki, J.; Grieb, P. Cladribine with or without prednisone in the treatment of previously treated and untreated B-cell chronic lymphocytic leukemia- updated results of the multicentre study of 378 patients. Br. J. Haematol. 2000, 108, 357–368. [Google Scholar] [CrossRef]
- Keating, M.J.; O'Brien, S.; Lerner, S.; Koller, C.; Beran, M.; Robertson, L.E.; Freireich, E.J.; Estey, E.; Kantarjian, H. Long term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regiments as initial therapy. Blood 1998, 92, 1165–1171. [Google Scholar]
- Piro, L.D.; Carrera, C.J.; Beutler, E.; Carson, D.A. 2-Chlorodeoxyadenosine: an effective new agent for the treatment of chronic lymphocytic leukemia. Blood 1988, 71, 1069–1073. [Google Scholar]
- Dillman, R.O. Pentostatin (Nipent) in the treatment of chronic lymphocyte leukemia and hairy cell leukemia. Expert Rev. Anticancer Ther. 2004, 4, 27–36. [Google Scholar] [CrossRef]
- Boogaerts, M.A. Oral fludarabine therapy in chronic lymphocytic leukemia-increased convenience. Hematology 2004, 5, 31–37. [Google Scholar] [CrossRef]
- O'Brien, S.M.; Kantarjian, H.M.; Cortes, J.; Beran, M.; Koller, C.A.; Giles, F.J.; Lerner, S.; Keating, M. Results of the fludarabine and cyclophosphamide combination regimen in chronic lymphocytic leukemia. J. Clin. Oncol. 2001, 19, 1414–1420. [Google Scholar]
- Hallek, M.; Schmitt, B.; Wilhelm, M.; Busch, R.; Kröber, A.; Fostitsch, H.P.; Sezer, O.; Herold, M.; Knauf, W.; Wendtner, C.M.; Kuse, R.; Freund, M.; Franke, A.; Schriever, F.; Nerl, C.; Döhner, H.; Thiel, E.; Hiddemann, W.; Brittinger, G.; Emmerich, B.; German Chronic Lymphocytic Leukaemia Study Group. Fludarabine plus cyclophosphamide is an efficient treatment for advanced chronic lymphocytic leukaemia (CLL): results of a phase II study of the German CLL Study Group. Br. J. Haematol. 2001, 14, 342–348. [Google Scholar]
- Eichhorst, B.F.; Busch, R.; Hopfinger, G.; Pasold, R.; Hensel, M.; Steinbrecher, C.; Siehl, S.; Jäger, U.; Bergmann, M.; Stilgenbauer, S.; Schweighofer, C.; Wendtner, C.M,; Döhner, H.; Brittinger, G.; Emmerich, B.; Hallek, M.; German CLL Study Group. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood 2006, 107, 885–891. [Google Scholar]
- Flinn, I.W.; Neuberg, D.S.; Grever, M.R.; Dewald, G.W.; Bennett, J.M.; Paietta, E.M.; Hussein, M.A.; Appelbaum, F.R.; Larson, R.A.; Moore, D.F., Jr.; Tallman, M,S. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J. Clin. Oncol. 2007, 25, 793–798. [Google Scholar] [CrossRef]
- Catovsky, D.; Richards, S.; Matutes, E.; Oscier, D.; Dyer, M.J.; Bezares, R.F.; Pettitt, A.R,; Hamblin, T.; Milligan, D.W.; Child, J.A.; Hamilton, M.S.; Dearden, C,E.; Smith, A.G.; Bosanquet, A.G.; Davis, Z.; Brito-Babapulle, V.; Else, M.; Wade, R.; Hillmen, P; UK National Cancer Research Institute (NCRI) Haematological Oncology Clinical Studies Group; NCRI Chronic Lymphocytic Leukaemia Working Group. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukemia (the LRF CLL4 Trial): a randomized controlled trial. Lancet 2007, 370, 230–239. [Google Scholar] [CrossRef]
- Montillo, M.; Tedeschi, A.; O'Brien, S.; Di Raimondo, F.; Lerner, S.; Ferrajoli, A.; Morra, E.; Keating, M.J. Phase II study of cladribine and cyclophosphamide in patients with chronic lymphocytic leukemia and prolymphocytic leukemia. Cancer 2003, 97, 114–120. [Google Scholar] [CrossRef]
- Robak, T.; Błoński, J.Z.; Kasznicki, M.; Góra-Tybor, J.; Dwilewicz-Trojaczek, J.; Stella-Hołowiecka, B.; Wołowiec, D. Cladribine combined with cyclophosphamide is highly effective in the treatment of chronic lymphocytic leukemia. Hematol. J. 2002, 3, 244–250. [Google Scholar] [CrossRef]
- Robak, T.; Blonski, J.Z.; Jamroziak, K.; Gora-Tybor, J.; Stella-Holowiecka, B.; Konopka, L.; Ceglarek, B.; Warzocha, K.; Seferynska, I.; Kloczko, J.; Piszcz, J.; Calbecka, M.; Kostyra, A.; Dwilewicz-Trojaczek, J.; Wiater, E.; Dmoszynska, A.; Kowal, M.; Zawilska, K.; Grzywacz, A.; Hellmann, A.; Mital, A.; Zduńczyk, A.; Dębowicz, J.; Kuliczkowski, K.; Potoczek, S.; Skotnicki, A.; Nowakowska-Domagala, M.; Lewandowski, K.; Sulek, K. Randomized comparison of cladribine plus cyclophosphamide with fludarabine plus cyclophosphamde in untreated patients with chronic lymphocytic leukemia: Report of the Polish Adult Leukemia Group (PALG-CLL3). Blood 2008, 112, 732a, (Abstract 2103). [Google Scholar]
- Weiss, M.A.; Maslak, P.G.; Jurcic, J.G.; Scheinberg, D.A.; Aliff, T.B.; Lamanna, N.; Frankel, S.R.; Kossman, S.E.; Horgan, D. Pentostatin and cyclophosphamide: an effective new regimen in previously treated patients with chronic lymphocytic leukemia. J. Clin. Oncol. 2003, 21, 1278–1284. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Keating, M.J.; Giles, F.J.; Wierda, W.G.; Ferrajoli, A.; Lerner, S.; Beran, M.; Andreeff, M.; Kantarjian, H.M.; O'Brien, S. Fludarabine and mitoxantrone for patients with chronic lymphocytic leukemia. Cancer 2004, 100, 2583–2591. [Google Scholar] [CrossRef]
- Bosch, F.; Ferrer, A.; López-Guillermo, A.; Giné, E.; Bellosillo, B.; Villamor, N.; Colomer, D.; Cobo, F.; Perales, M.; Esteve, J.; Altés, A.; Besalduch, J.; Ribera, J.M.; Montserrat, E. For the GELCAB (Grup per l'Estudi dels Limfomes a Catalunya i Balears). Fludarabine, cyclophosphamide and mitoxantrone in the treatment of resistant or relapsed chronic lymphocytic leukemia. Br. J. Haematol. 2002, 119, 976–984. [Google Scholar] [CrossRef]
- Robak, T.; Góra-Tybor, J.; Lech-Marańda, E.; Błoński, J.Z.; Kasznicki, M. Cladribine in combination with mitoxantrone and cyclophosphamide (CMC) in the treatment of heavily pre-treated patients with advanced indolent lymphoid malignancies. Eur. J. Haematol. 2001, 66, 188–194. [Google Scholar] [CrossRef]
- Robak, T.; Błoński, J.Z.; Kasznicki, M.; Góra – Tybor, J.; Dwilewicz-Trojaczek, J.; Boguradzki, P.; Konopka, L.; Ceglarek, B.; Sułek, J.; Kuliczkowski, K.; Wołowiec, D.; Stella-Hołowiecka, B.; Skotnicki, AB.; Nowak, W.; Moskwa-Sroka, B.; Dmoszyńska, A.; Calbecka, M. Cladribine combined with cyclophosphamide and mitoxantrone as front-line therapy in chronic lymphocytic leukemia. Leukemia 2001, 15, 1510–1516. [Google Scholar] [CrossRef]
- Robak, T.; Blonski, J.Z.; Gora-Tybor, J.; Jamroziak, K.; Dwilewicz-Trojaczek, J.; Tomaszewska, A.; Konopka, L.; Ceglarek, B.; Dmoszynska, A.; Kowal, M.; Kloczko, J.; Stella-Holowiecka, B.; Sulek, K.; Calbecka, M.; Zawilska, K.; Kuliczkowski, K.; Skotnicki, A.B.; Warzocha, K.; Kasznicki, M.; Polish Leukemia Group (PALG CLL2). Cladribine alone and in combination with cyclophosphamide or cyclophosphamide plus mitoxantrone in the treatment of progressive chronic lymphocytic leukemia: report of a prospective, multicenter, randomized trial of the Polish Adult Leukemia Group (PALG CLL2). Blood. 2006, 108, 473–479. [Google Scholar] [CrossRef]
- Robak, T. Novel monoclonal antibodies for chronic lymphocytic leukemia. Curr. Cancer Drug Targets 2008, 8, 156–171. [Google Scholar] [CrossRef]
- Keating, M.J.; O'Brien, S.; Albitar, M.; Lerner, S.; Plunkett, W.; Giles, F.; Andreeff, M.; Cortes, J.; Faderl, S.; Thomas, D.; Koller, C.; Wierda, W.; Detry, M.A.; Lynn, A.; Kantarjian, H. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide and rituximab as initial therapy for chronic lymphocytic leukemia. J. Clin. Oncol. 2005, 23, 4079–4088. [Google Scholar] [CrossRef]
- Wierda, W.; O'Brien, S.; Wen, S.; Faderl, S.; Garcia-Manero, G.; Thomas, D.; Do, K.A.; Cortes, J.; Koller, C.; Beran, M.; Ferrajoli, A.; Giles, F.; Lerner, S.; Albitar, M.; Kantarjian, H.; Keating, M. Chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab for relapsed and refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2005, 23, 4070–4078. [Google Scholar] [CrossRef]
- Byrd, J.C.; Rai, K.; Peterson, B.L.; Appelbaum, F.R.; Morrison, V.A.; Kolitz, J.E.; Shepherd, L.; Hines, J.D.; Schiffer, C.A.; Larson, R.A. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective analysis of CALGB 9712 and CALGB 9011. Blood 2005, 105, 49–53. [Google Scholar] [CrossRef]
- Hallek, M.; Fingerle-Rowson, G.; Fink, A.M.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; von Gruenhagen, U.; Bergmann, M.A.; Catalano, J.; Zinzani, P.L.; Caligaris Cappio, F.; Seymour, F.J.; Berrebi, A.; Jaeger, U.; Cazin, B.; Trneny, M.; Westermann, A.; Wendtner, C. MD1; Eichhorst, B.F.; Staib, P.; Boettcher, S.; Ritgen, M.; Stilgenbauer, S.; Mendila, M.; Kneba, M.; Döhner, H.; Fischer, K. Immunochemotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) versus fludarabine and cyclophosphamide (FC) improves response rates and progression-free survival (PFS) of previously untreated patients (pts) with advanced chronic lymphocytic leukemia (CLL). Blood 2008, 112, 125a, (Abstract 325). [Google Scholar]
- Robak, T.; Moiseev, S.; Dmoszynska, A.; Solal-Céligny, P.; Warzocha, K.; Javier Loscertales, J.; Catalano, J. MD.; Afanasiev, B.V.; Larratt, L.; Geisler, C.; Montillo, M.; Ganly, P.; Dartigeas, C.; Rosta, A.; Janssens, A.; Mendila, M.; Maurer, J.; Wenger, M.K. Rituximab, fudarabine, and cclophosphamide (R-FC) polongs pogression fee survival in relapsed or refractory chronic lymphocytic leukemia (CLL) cmpared with FC aone: Final results from the international randomized phase III REACH tial. Blood 2008, 112, LBA-1, (Abstract 157420). [Google Scholar] [CrossRef]
- Robak, T.; Smolewski, P.; Cebula, B.; Grzybowska-Izydorczyk, O.; Błoński, J.Z. Rituximab plus cladribine with or without cyclophosphamide in patients with relapsed or refractory chronic lymphocytic leukemia. Eur. J. Haematol. 2007, 79, 107–113. [Google Scholar] [CrossRef]
- Robak, T.; Smolewski, P.; Cebula, B.; Szmigielska-Kaplon, A.; Chojnowski, K.; Blonski, J.Z. Rituximab combined with cladribine or with cladribine and cyclophosphamide in heavily pretreated patients with indolent lymphoproliferative disorders and mantle cell lymphoma. Cancer 2006, 107, 1542–1550. [Google Scholar] [CrossRef]
- Absi, A.; Hsi, E.; Kulaycio, M. Prolymphocytic leukemia. Curr. Treat Options Oncol. 2005, 6, 197–208. [Google Scholar] [CrossRef]
- Robak, T.; Robak, P. Current treatment options in prolymphocytic leukemia. Med. Sci. Monit. 2007, 13, RA 69–80. [Google Scholar]
- Krishnan, B.; Matutes, E.; Dearden, C. Prolymphocytic leukemias. Semin Oncol. 2006, 33, 257–263. [Google Scholar] [CrossRef]
- Palomera, L.; Domingo, J.M.; Agullo, J.A.; Romero, M. Complete remission in T-cell prolymphocytic leukemia with 2-chlorodeoxyadenosine (Letter). J.Clin. Oncol. 1995, 13, 1284–1285. [Google Scholar]
- Saven, A.; Lee, T.; Schlutz, M.; Jacobs, A.; Ellison, D.; Longmire, R.; Piro, L. Major activity of cladribine in patients with de novo B-cell prolymphocytic leukemia. J. Clin. Oncol. 1997, 15, 37–43. [Google Scholar]
- Mercieca, J.; Matutes, E.; Dearden, C.; MacLennan, K.; Catovsky, D. The role of pentostatin in the treatment of T-cell malignancies: analysis of response rate in 145 patients according to disease subtype. J. Clin. Oncol. 1994, 12, 2588–2593. [Google Scholar]
- Kantarjian, H.M.; Childs, C.; O'Brien, S.; Huh, Y.; Beran, M.; Schachner, J.; Koller, C.; Keating, M.J. Efficacy of fludarabine, a new adenine nucleoside analogue, in patients with prolymphocytic leukemia and the prolymphocytoid variant of chronic lymphocytic leukemia. Am. J. Med. 1991, 90, 223–228. [Google Scholar] [CrossRef]
- Dohner, H.; Ho, A.D.; Thaler, J.; Stryckmans, P.; Sonneveld, P.; de Witte, T.; Lechner, K.; Lauria, F; Bodewadt-Radzun, S.; Suciu, S. Pentostatin in prolymphocytic leukemia: phase II trial of the European Organization for Research and Treatment of Cancer Leukemia Cooperative Study Group. J. Natl. Cancer Inst. 1993, 85, 658–662. [Google Scholar] [CrossRef]
- Doorduijn, J.K.; Michiels, J.J. Effectiveness of fludarabine in end-stage prolymphocytic leukemia. Leukemia 1994, 8, 1439. [Google Scholar]
- Jeha, S.; Razzouk, B.; Rytting, M.E.; Gaynon, P.S.; Kadota, R.; Rheingold, S.; Luchtman-Jones, L.; Shen, V.; Arceci, R.J.; Fernandez, M.; Weitman, S.; Steinherz, P.G. Phase II trials of clofarabine in relapsed or refractory pediatric leukemia. Blood 2004, 104, 196a, (Abstract 684). [Google Scholar]
- Jeha, S.; Gaynon, P.S.; Steinherz, P.; Kadota, R.; Bomgaars, L.; Razzouk, B.; Luchtman-Jones, L.; Altman, A.; Rheingold, S.; Ritchey, A.K.; Shen, V.; Weiss, J.; Chan, K.W.; Craig, A.; Arceci, R.J. A Phase II, open-label study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood 2003, 102, 881a, (Abstract 3277). [Google Scholar] [CrossRef]
- Jeha, S.; Steinherz, P.; Gaynon, P.S.; Rheingold, S.; Razzouk, B.; Ritchey, A.K.; Altman, A.; Albano, E.; Stine, K.; Chan, K.W.; Weiss, J.; Craig, A.; Arceci, R.J. A Phase II, open-label study of clofarabine in pediatric patients with refractory or relapsed acute myelogenous leukemia. Blood 2003, 102, 617a, (Abstract 2278). [Google Scholar]
- Jeha, S.; Gaynon, P.S.; Razzouk, B.I.; Franklin, J.; Kadota, R.; Shen, V.; Luchtman-Jones, L.; Rytting, M.; Bomgaars, L.R.; Rheingold, S.; Ritchey, K.; Albano, E.; Arceci, R.J.; Goldman, S.; Griffin, T.; Altman, A.; Gordon, B.; Steinherz, L.; Weitman, S.; Steinherz, P. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J. Clin. Oncol. 2006, 24, 1917–1923. [Google Scholar] [CrossRef]
- Gidwani, P.; Ramesh, K.H.; Liu, Y.; Kolb, E.A. The comparison of clofarabine and cytarabine in pediatric relapsed acute lymphoblastic leukemia: a case report. Chemotherapy 2008, 54, 120–124. [Google Scholar] [CrossRef]
- Karp, J.E.; Ricklis, R.M.; Balakrishnan, K.; Briel, J.; Greer, J.; Gore, S.D.; Smith, B.D.; McDevitt, M.A.; Carraway, H.; Levis, M.J.; Gandhi, V. A phase 1 clinical-laboratory study of clofarabine followed by cyclophosphamide for adults with refractory acute leukemias. Blood 2007, 110, 1762–1769. [Google Scholar] [CrossRef]
- Cohen, M.H.; Johnson, J.R.; Justice, R.; Pazdur, R. FDA drug approval summary: nelarabine (Arranon) for the treatment of T-cell lymphoblastic leukemia/lymphoma. Oncologist. 2008, 13, 709–714. [Google Scholar] [CrossRef]
- Kurtzberg, J.; Ernst, T.J.; Keating, M.J.; Gandhi, V.; Hodge, J.P.; Kisor, D.F.; Lager, J.J.; Stephens, C.; Levin, J.; Krenitsky, T.; Elion, G.; Mitchell, B.S. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J. Clin. Oncol. 2005, 23, 3396–3403. [Google Scholar] [CrossRef]
- Berg, S.L.; Blaney, S.M.; Devidas, M.; Lampkin, T.A.; Murgo, A.; Bernstein, M.; Billett, A.; Kurtzberg, J.; Reaman, G.; Gaynon, P.; Whitlock, J.; Krailo, M.; Harris, M.B. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children's Oncology Group. J. Clin. Oncol. 2005, 23, 3376–3403. [Google Scholar] [CrossRef]
- Furman, R.R.; Iosava, G.; Isola, L.; Ravandi, F.; Zodelava, M.; Bennett, J.C.; Kilpatrick, J.M.; Bantia, S. Forodesine (FodosineTM), a PNP inhibitor active in relapsed or refractory T-cell leukemia patients (Phase II study). Blood 2005, 106, 259a, (Abstract 881). [Google Scholar]
- Stelljes, M.; Kienast, J.; Berning, B.; Gokbuget, M.; Hoelzer, D.; Silling, G.; Berdel, W.E.; Dahl, G.V.H.; Schissel, D.; Hemenway, M.; Gore, L. Forodesine in patients with refractory/relapsed T-ALL can induce prolonged stable remission with minimal toxicity before and after allogeneic hematopoietic stem cell transplantation. Blood 2006, 108, 427b, (Abstract 5340). [Google Scholar]
- Furman, R.R.; Gandhi, V.V.; Bennett, J.C.; Bantia, S.; Kilpatrick, J.M. Intravenous Forodesine (BCX-1777), a novel purine nucleoside phosphorylase (PNP) inhibitor, demonstrates clinical activity in phase I/II studies in patients with B-Cell acute lymphoblastic leukemia. Blood 2004, 104, 750a, (Abstract 2743). [Google Scholar]
- Ritchie, E.; Gore, L.; Roboz, G.J.; Feldman, E.; Ravandi, F.; Furman, R. Phase II study of forodesine, a PNP inhibitor, in patients with relapsed or refractory B-lineage acute lymphoblastic leukemia. Blood 2006, 108, 533a, (Abstract 1881). [Google Scholar]
- Tondini, C.; Balzarotti, M.; Rampinelli, J.; Valagussa, P.; Luoni, M.; DePaoli, A.; Santoro, A.; Bonadonna, G. Fludarabine and cladribine in relapsed/refractory low-grade non-Hodgkin's lymphoma: a phase II randomized study. Ann. Oncol. 2000, 11, 231–233. [Google Scholar]
- Robak, T.; Góra-Tybor, J.; Krykowski, E.; Walewski, J.A.; Borawska, A.; Pluzanska, A.; Potemski, P.; Hellmann, A.; Zaucha, J.M.; Konopka, L.; Ceglarek, B.; Durzynski, T.; Sikorska, A`; Michalak, K.; Urasinski, J.; Opalinska, J.; Dmoszynska, A.; Adamczyk-Cioch, M.B.; Kuratowska, Z`; Dwilewicz-Trojaczek, J.; Boguradzki, P.; Deren, M`; Maj, S.; Grieb, P. Activity of 2-chlorodeoxyadenosine (Cladribine) in 2-hour intravenous infusion in 94 previously treated patients with low grade non-Hodgkin's lymphoma. Leuk. Lymphoma. 1997, 26, 99–105. [Google Scholar] [CrossRef]
- Tulpule, A.; Schiller, G.; Harycy-Buchman, L.; Tulpule, A.; Schiller, G.; Harvey-Buchanan, L.A.; Lee, M.; Espina, B.M.; Khan, A.U.; Boswell, W.; Nathwani, B.; Levine, A.M. Cladribine in the treatment of advanced relapsed or refractory low and intermediate grade non-Hodgkin's lymphoma. Cancer 1998, 83, 2370–2376. [Google Scholar] [CrossRef]
- Blum, K.A.; Johnson, J.L.; Niedzwiecki, D.; Piro, L.D.; Saven, A.; Peterson, B.A.; Byrd, J.C.; Cheson, B.D. Cancer and Leukemia Group B Study 9153. Prolonged follow-up after initial therapy with 2-chlorodeoxyadenosine in patients with indolent non-Hodgkin lymphoma: results of Cancer and Leukemia Group B Study 9153. Cancer 2006, 107, 2817–2825. [Google Scholar] [CrossRef]
- Kong, L.R.; Huang, C.F.; Hakimian, D.; Variakojis, D.; Klein, L.; Kuzel, T.M.; Gordon, L.I.; Zanzig, C.; Wollins, E.; Tallman, M.S. Long term follow-up and late complications of 2-chlorodeoxyadenosine in previously treated, advanced, indolent non-Hodgkin's lymphoma. Cancer 1998, 82, 957–964. [Google Scholar] [CrossRef]
- Saven, A.; Emanuele, S.; Kosty, M.; Kozioł, J.; Ellison, D.; Piro, L. 2-Chlorodeoxyadenosine activity in patients with untreated, indolent non-Hodgkin's lymphoma. Blood 1995, 86, 1710–1716. [Google Scholar]
- Fridrik, M.A.; Jager, G.; Kienzer, H.R.; Hausmaninger, H.; Oppitz, P.; Krieger, O.; Zabernigg, A.; Lang, A.; Neubauer, M.; Weidinger, G.; Schiller, L.; Seewann, H.L.; Chott, A.; Linkesch, W. Efficacy and toxicity of 2-Chlorodeoxyadenosine (Cladribine)--2 h infusion for 5 days--as first-line treatment for advanced low grade non-Hodgkin's lymphoma. Eu.r J. Cancer 1998, 34, 1560–1564. [Google Scholar] [CrossRef]
- Liliemark, J.; Martinsson, U.; Cavallin-Ståhl, E.; Svedmyr, E.; Porwit, A.; Strömberg, M.; Juliusson, G. Cladribine for untreated or early low-grade non-Hodgkin's lymphoma. Leuk. Lymphoma 1998, 30, 573–581. [Google Scholar]
- Robak, T.; Góra-Tybor, J.; Urbańska-Ryś, H.; Krykowski, E. Combination regimen of 2-chlorodeoxyadenosine (cladribine), mitoxantrone and dexamethasone (CMD) in the treatment of refractory and recurrent low grade non-Hodgkin's lymphoma. Leuk. Lymphoma. 1999, 32, 359–363. [Google Scholar]
- Van den Neste, E.; Louviaux, I.; Michaux, J.L.; Sonet, A.; Bosly, A.; Doyen, C.; Mineur, P.; Andre, M.; Straetmans, N.; Coche, E.; Venet, C.; Duprez, T.; Ferrant, A. Phase I/II study of 2-chloro-2'-deoxyadenosine with cyclophosphamide in patients with pretreated B cell chronic lymphocytic leukemia and indolent non-Hodgkin's lymphoma. Leukemia 2000, 14, 1136–1142. [Google Scholar] [CrossRef]
- Armitage, J.O.; Tobinai, K.; Hoelzer, D.; Rummel, M.J. Treatment of indolent non-Hodgkin's lymphoma with cladribine as single-agent therapy and in combination with mitoxantrone. Int. J.Hematol. 2004, 79, 311–321. [Google Scholar] [CrossRef]
- Riccioni, R.; Caracciolo, F.; Galimberti, S.; Cecconi, N.; Petrini, M. Low dose 2-CdA schedule activity in splenic marginal zone lymphomas. Hematol. Oncol. 2003, 21, 163–168. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Tani, M.; Fanti, S.; Stefoni, V.; Musuraca, G.; Vitolo, U.; Perrotti, A.; Fina, M.; Derenzini, E.; Baccarani, M. A phase 2 trial of fludarabine and mitoxantrone chemotherapy followed by yttrium-90 ibritumomab tiuxetan for patients with previously untreated, indolent, nonfollicular, non-Hodgkin lymphoma. Cancer 2008, 112, 856–862. [Google Scholar] [CrossRef]
- Rummel, M.J.; Chow, K.U.; Karakas, T.; Jäger, E.; Mezger, J.; von Grünhagen, U.; Schalk, K.P.; Burkhard, O.; Hansmann, M.L.; Ritzel, H.; Bergmann, L.; Hoelzer, D.; Mitrou, P.S. Reduced dose cladribine (2-CdA) plus mitoxantrone is effective in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. Eur. J. Cancer 2002, 38, 1739–1746. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Magagnoli, M.; Bandandi, M.; Tani, M.; Stefoni, V.; Cellini, C.; Poggi, S.; Piccioli, M.; Pileri, S.; Tura, S. Long-term follow-up of hairy cell leukemia patients treated with 2-chlorodeoxyadenosine. Haematologica 2000, 85, 922–925. [Google Scholar]
- Hagenbeek, A.; Eghbali, H.; Monfardini, S.; Resegotti, E.; Hoskin, J.; de Wolf Peeters, C.; Mc Lennan, K.; Staab-Renner, E.; Schott, A.; Teodorovic, I.; Negrouk, A.; van Glabbeke, M.; Marcus, R. Fludarabine compared with CVP chemotherapy in newly diagnosed patients with stages III and IV low grade malignant non-Hodgkin’s lymphoma. Blood 2001, 98, 843a, (Abstract 3501). [Google Scholar]
- Cabanillas, F. Purine nucleoside analogs in indolent non-Hodgkin's lymphoma. Oncology 2000, 14, 13–15. [Google Scholar]
- Klasa, R.J.; Meyer, R.M.; Shustik, C.; Sawka, C.A.; Smith, A.; Guevin, R.; Maksymiuk, A.; Rubinger, M.; Samosh, M.; Laplante, S.; Grenier, J.F. Randomized phase III study of fludarabine phosphate versus cyclophosphamide, vincristine, and prednisone in patients with recurrent low-grade non-Hodgkin's lymphoma previously treated with an alkylating agent or alkylator-containing regimen. J. Clin. Oncol. 2002, 20, 4649–4654. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Stefoni, V.; Musuraca, G.; Tani, M.; Alinari, L.; Gabriele, A.; Marchi, E.; Pileri, S.; Bacarani, M. Fludarabine-containing chemotherapy as frontline treatment of nongastrointestinal mucosa-associated lymphoid tissue lymphoma. Cancer 2004, 100, 2190–2194. [Google Scholar] [CrossRef]
- Jäger, G.; Neumeister, P.; Brezinschek, R.; Hinterleitner, T.; Fiebiger, W.; Penz, M.; Neumann, H.J.; Mlineritsch, B.; De Santis, M.; Quehenberger, F.; Chott, A.; Beham-Schmid, C.; Höfler, G.; Linkesch, W.; Raderer, M. Treatment of extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type with cladribine: a phase II study. J. Clin. Oncol. 2002, 20, 3872–3877. [Google Scholar] [CrossRef]
- Robak, T.; Lech-Maranda, E.; Janus, A.; Blonski, J.; Wierzbowska, A.; Gora-Tybor, J. Cladribine combined with cyclophosphamide and mitoxantrone is an active salvage therapy in advanced non-Hodgkin's lymphoma. Leuk Lymphoma. 2007, 48, 1092–1101. [Google Scholar] [CrossRef]
- Forstpointner, R.; Dreyling, M.; Repp, R.; Hermann, S.; Hänel, A.; Metzner, B.; Pott, C.; Hartmann, F.; Rothmann, F.; Rohrberg, R.; Böck, H.P.; Wandt, H.; Unterhalt, M.; Hiddemann, W.; German Low-Grade Lymphoma Study Group. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2004, 104, 3064–3071. [Google Scholar] [CrossRef]
- Cohen, B.J.; Moskowitz, C.; Straus, D.; Noy, A.; Hedrick, E.; Zelenetz, A. Cyclophosphamide/fludarabine (CF) is active in the treatment of mantle cell lymphoma. Leuk Lymphoma 2001, 42, 1015–1022. [Google Scholar] [CrossRef]
- Thomas, D.W.; Owen, R.G.; Johnson, S.A.N.; Hillmen, P.; Seymour, J.F.; Wolf, M.M.; Rule, S.A.J. Superior quality and duration of response among patients with mantle cell lymphoma treated with fludarabine and cyclophosphamide with or without rituximab compared with prior responses to CHOP. Leuk. Lymphoma 2005, 46, 549–552. [Google Scholar] [CrossRef]
- Robak, T.; Smolewski, P.; Cebula, B.; Bloński, J.Z. Rituximab combined with cladribine or with cladribine and cyclophosphamide in heavily pretreated patients with indolent lymphoproliferative disorders and mantle cell lymphoma. Cancer 2006, 107, 1542–1550. [Google Scholar] [CrossRef]
- Saven, A.; Burian, C.; Kozioł, J.A.; Piro, L.D. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood 1998, 92, 1918–1926. [Google Scholar]
- Anaissie, E.J.; Kontoyiannis, D.P.; O’Brien, S.; Kantarjian, H.; Robertson, L.; Lerner, S.; Keating, M.J. Infections in patients with chronic lymphocytic leukemia treated with fludarabine. Ann.Intern. Med. 1998, 129, 559–566. [Google Scholar] [CrossRef]
- Van den Neste, E.; Delannoy, A.; Vandercam, B.; Bosly, A.; Ferrant, A.; Mineur, P.; Montfort, L.; Martiat, P.; Straetmans, N.; Filleul, B.; Michaux, J.L. Infectious complications after 2-chlorodeoxyadenosine therapy. Eur. J. Haematol. 1996, 56, 235–240. [Google Scholar]
- Samonis, G.; Kontoyiannis, D.P. Infectious complications of purine analog therapy. Curr. Opin. Infect. Dis. 2001, 14, 409–413. [Google Scholar] [CrossRef]
- O’Brien, S. Infections complications of nucleoside analogs. In Hematology; the American Society of Hematology: USA, 1999; pp. 536–542. [Google Scholar]
- Anaissie, E.J.; Kontoyiannis, D.P.; O'Brien, S.; Kantarjian, H.; Robertson, L.; Lerner, S.; Keating, M.J. Infections in patients with chronic lymphocytic leukemia treated with fludarabine. Ann. Intern. Med. 1998, 129, 559–566. [Google Scholar] [CrossRef]
- Perkins, J.G.; Flynn, J.M.; Howard, R.S.; Byrd, J.C. Frequency and type of serious infections in fludarabine –refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma : implications for clinical trials in this patients population. Cancer 2002, 94, 2033–2039. [Google Scholar] [CrossRef]
- Cheson, B.D. Infectious and immunosuppressive complications of purine analog therapy. J. Clin. Oncol. 1995, 13, 2431–2448. [Google Scholar]
- O'Brien, S.; Kantarjian, H.; Beran, M.; Koller, C.; Talpaz, M.; Lerner, S.; Keating, M.J. Fludarabine and granulocyte colony stimulating factor (G-CSF) in patients with chronic lymphocytic leukemia. Leukemia 1997, 11, 1631–1635. [Google Scholar]
- Sudhoff, T.; Arning, M.; Schneider, W. Prophylactic strategies to meet infectious complications in fludarabine treated CLL. Leukemia 1997, 11, S38–S41. [Google Scholar]
- Byrd, J.C.; Hargis, J.B.; Kester, K.E.; Hospenthal, D.R.; Knutnson, S.W.; Diehl, L.F. Opportunistic pulmonary infections with fludarabine in previously treated patients with low-grade lymphoid malignancies: a role for Pneumocystic carini pneumonia prophylaxis. Am J, Hematol. 1995, 49, 135–142. [Google Scholar]
- Samonis, G.; Kontoyiannis, D.P. Infections complications of purine analog therapy. Curr. Opinion Infect. Dis. 2001, 14, 409–413. [Google Scholar]
- Mauro, F.R.; Foa, R.; Cerreti, R.; Giannarelli, D.; Coluzzi, S.; Mandelli, F.; Girreli, G. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: clinical, therapeutic, and prognostic features. Blood 2000, 95, 2786–2792. [Google Scholar]
- Kroft, S.H.; Tallman, M.S.; Shaw, J.M.; Thangavelu, M.; Peterson, L.C. Myelodysplasia following treatment of chronic lymphocytic leukemia (CLL) with 2-chlorodeoxyadenosine (2-CdA). Leukemia 1997, 11, 170. [Google Scholar]
- Van Den Neste, E.; Louviaux, I.; Michaux, J.L.; Delanoy, A.; Michaux, L.; Hagemeijer, A.; Scheiff, J.M.; Bosly, A.; Stractmans, N.; Ferrant, A. Myelodysplastic syndrome with monosomy 5 and/or 7 following therapy with 2-chloro-2'-deoxyadenosine. Br. J.Haematol. 1999, 105, 268–270. [Google Scholar]
- Robak, T. Second malignancies and Richter's syndrome in patients with chronic lymphocytic leukemia. Haematology 2004, 9, 387–400. [Google Scholar] [CrossRef]
- Cheson, B.D.; Vena, D.A.; Barrett, J.; Freidlin, B. Second malignancies as a consequence of nucleoside analog therapy for chronic lymphoid leukemias. J.Clin. Oncol. 1999, 17, 2454–2460. [Google Scholar]
- Robak, T.; Błoński, J.Z.; Góra-Tybor, J.; Kasznicki, M.; Konopka, L.; Ceglarek, B.; Komarnicki, M.; Lewandowski, K.; Hellmann, A.; Lewandowski, K.; Moskwa, A.; Dmoszyńska, A.; Sokołowska, B.; Dwilewicz-Trojaczek, A.; Tomaszewska, A.; Sułek, K.; Całbecka, M. Second malignancies and Richter's syndrome in patients with chronic lymphocytic leukaemia treated with cladribine. Eur.J. Cancer 2004, 40, 383–389. [Google Scholar] [CrossRef]
- Sample availability: Not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Robak, T.; Korycka, A.; Lech-Maranda, E.; Robak, P. Current Status of Older and New Purine Nucleoside Analogues in the Treatment of Lymphoproliferative Diseases. Molecules 2009, 14, 1183-1226. https://doi.org/10.3390/molecules14031183
Robak T, Korycka A, Lech-Maranda E, Robak P. Current Status of Older and New Purine Nucleoside Analogues in the Treatment of Lymphoproliferative Diseases. Molecules. 2009; 14(3):1183-1226. https://doi.org/10.3390/molecules14031183
Chicago/Turabian StyleRobak, Tadeusz, Anna Korycka, Ewa Lech-Maranda, and Pawel Robak. 2009. "Current Status of Older and New Purine Nucleoside Analogues in the Treatment of Lymphoproliferative Diseases" Molecules 14, no. 3: 1183-1226. https://doi.org/10.3390/molecules14031183
APA StyleRobak, T., Korycka, A., Lech-Maranda, E., & Robak, P. (2009). Current Status of Older and New Purine Nucleoside Analogues in the Treatment of Lymphoproliferative Diseases. Molecules, 14(3), 1183-1226. https://doi.org/10.3390/molecules14031183



