1
General Chemistry (ALGC), Materials Modelling Group, Vrije Universiteit Brussels, 1050 Brussels, Belgium
2
Grupo de Química Computacional y Teórica (QCT-USFQ), Departamento de Ingeniería Química, Universidad San Francisco de Quito (USFQ), Diego de Robles y Vía Interoceánica, Quito 17-1200-841, Ecuador
3
Instituto de Simulación Computacional (ISC-USFQ), Departamento de Ingeniería Química, Universidad San Francisco de Quito (USFQ), Diego de Robles y Vía Interoceánica, Quito 17-1200-841, Ecuador
4
Kidney Stone Clinic, Nephrology Department, Centre Hospitalier Universitaire, Brugmann Hospital, 1020 Brussels, Belgium
5
Faculty of Medicine, Université Libre de Bruxelles (ULB), 1050 Brussels, Belgium
6
Nephrology Department, Universitair Ziekenhuis Brussel, Vrije Universiteit Brussel, 1090 Brussels, Belgium
7
Sorbonne Universités-UPMC Univ. Paris 06, UMR S 1155, 75020 Paris, France
8
INSERM, UMR S 1155, 75020 Paris, France
9
Explorations Fonctionnelles Multidisciplinaires, AP-HP, Hôpital Tenon, 75020 Paris, France
10
Institut de Chimie Physique, UMR CNRS 8000, Université Paris Saclay, Bâtiment 350, CEDEX, 91405 Orsay, France
11
Laboratoire de Physique des Solides, UMR CNRS 8502, Université Paris-Saclay, Bâtiment 510, CEDEX, 91405 Orsay, France
†
Current address: Center for Molecular Modeling (CMM), Ghent University, Technologiepark-Zwijnaarde 46, 9052 Zwijnaarde, Belgium.
add
Show full affiliation list
Nanomaterials 2021, 11(7), 1763; https://doi.org/10.3390/nano11071763 - 6 Jul 2021
Cited by 11 | Viewed by 3864
Abstract
Limiting gastrointestinal oxalate absorption is a promising approach to reduce urinary oxalate excretion in patients with idiopathic and enteric hyperoxaluria. Phosphate binders, that inhibit gastrointestinal absorption of dietary phosphate by the formation of easily excretable insoluble complexes, are commonly used as a treatment
[...] Read more.
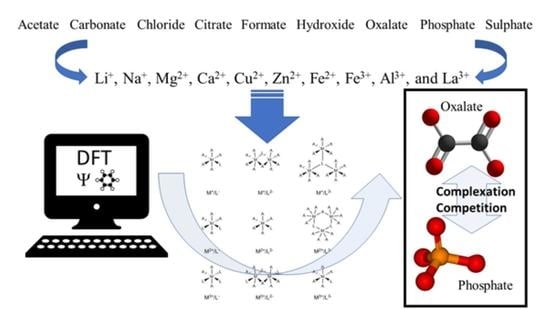
Limiting gastrointestinal oxalate absorption is a promising approach to reduce urinary oxalate excretion in patients with idiopathic and enteric hyperoxaluria. Phosphate binders, that inhibit gastrointestinal absorption of dietary phosphate by the formation of easily excretable insoluble complexes, are commonly used as a treatment for hyperphosphatemia in patients with end-stage renal disease. Several of these commercially available phosphate binders also have affinity for oxalate. In this work, a series of metallic cations (Li+, Na+, Mg2+, Ca2+, Fe2+, Cu2+, Zn2+, Al3+, Fe3+ and La3+) is investigated on their binding affinity to phosphate and oxalate on one side and anionic species that could be used to administer the cationic species to the body on the other, e.g., acetate, carbonate, chloride, citrate, formate, hydroxide and sulphate. Through quantum chemical calculations, the aim is to understand the competition between the different complexes and propose possible new and more efficient phosphate and oxalate binders.
Full article
(This article belongs to the Special Issue Theoretical Calculation and Molecular Modeling of Nanomaterials)
▼
Show Figures