Pharmaceutics 2019, 11(11), 589; https://doi.org/10.3390/pharmaceutics11110589 - 8 Nov 2019
Cited by 26 | Viewed by 5337
Abstract
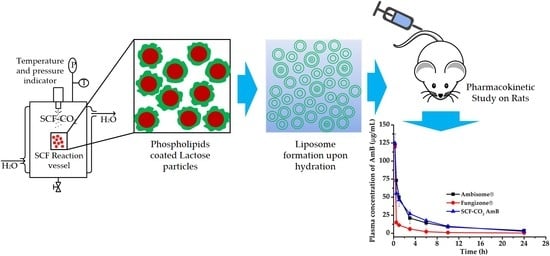
Here, we aimed to prepare and optimize liposomal amphotericin B (AmB) while using the supercritical fluid of carbon dioxide (SCF-CO2) method and investigate the characteristics and pharmacokinetics of the SCF-CO2-processed liposomal AmB. Liposomes containing phospholipids, ascorbic acid (vit C),
[...] Read more.
Here, we aimed to prepare and optimize liposomal amphotericin B (AmB) while using the supercritical fluid of carbon dioxide (SCF-CO2) method and investigate the characteristics and pharmacokinetics of the SCF-CO2-processed liposomal AmB. Liposomes containing phospholipids, ascorbic acid (vit C), and cholesterol were prepared by the SCF-CO2 method at an optimized pressure and temperature; conventional liposomes were also prepared using the thin film hydration method and then compared with the SCF-CO2-processed-liposomes. The optimized formulation was evaluated by in vitro hemolysis tests on rat erythrocytes and in vivo pharmacokinetics after intravenous administration to Sprague-Dawley rats and compared with a marketed AmB micellar formulation, Fungizone®, and a liposomal formulation, AmBisome®. The results of the characterization studies demonstrated that the SCF-CO2-processed-liposomes were spherical particles with an average particle size of 137 nm (after homogenization) and drug encapsulation efficiency (EE) was about 90%. After freeze-drying, mean particle size, EE, and zeta potential were not significantly changed. The stability study of the liposomes showed that liposomal AmB that was prepared by the SCF method was stable over time. In vivo pharmacokinetics revealed that the SCF-CO2-processed-liposomes were bioequivalent to AmBisome®; the hemolytic test depicted less hematotoxicity than Fungizone®. Therefore, this method could serve as a potential alternative for preparing liposomal AmB for industrial applications.
Full article
(This article belongs to the Special Issue Supercritical Fluid and Pharmaceutical Applications)
►
Show Figures