1
Department of Physics, University of Peshawar, Peshawar 25120, Pakistan
2
Department of Physics, Abdul Wali Khan University, Mardan 23200, Pakistan
3
Department of Chemical Engineering, NFC Institute of Engineering & Technology, Multan 60000, Pakistan
4
Department of Nano Energy Engineering, Pusan National University, Busan 46241, Republic of Korea
Molecules 2023, 28(7), 3252; https://doi.org/10.3390/molecules28073252 - 5 Apr 2023
Cited by 13 | Viewed by 2922
Abstract
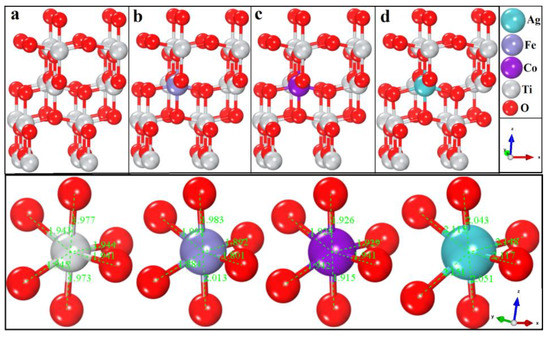
Titanium dioxide (TiO2) has been investigated for solar-energy-driven photoelectrical water splitting due to its suitable band gap, abundance, cost savings, environmental friendliness, and chemical stability. However, its poor conductivity, weak light absorption, and large indirect bandgap (3.2 eV) has limited its
[...] Read more.
Titanium dioxide (TiO2) has been investigated for solar-energy-driven photoelectrical water splitting due to its suitable band gap, abundance, cost savings, environmental friendliness, and chemical stability. However, its poor conductivity, weak light absorption, and large indirect bandgap (3.2 eV) has limited its application in water splitting. In this study, we precisely targeted these limitations using first-principle techniques. TiO2 only absorbs near-ultraviolet radiation; therefore, the substitution (2.1%) of Ag, Fe, and Co in TiO2 significantly altered its physical properties and shifted the bandgap from the ultraviolet to the visible region. Cobalt (Co) substitution in TiO2 resulted in high absorption and photoconductivity and a low bandgap energy suitable for the reduction in water without the need for external energy. The calculated elastic properties of Co-doped TiO2 indicate the ductile nature of the material with a strong average bond strength. Co-doped TiO2 exhibited fewer microcracks with a mechanically stable composition.
Full article
(This article belongs to the Special Issue Molecular Simulations of Energy Materials)
▼
Show Figures