Review of Animal Models of Prostate Cancer Bone Metastasis
Abstract
:1. Introduction
2. Prostate Cancer in Dogs
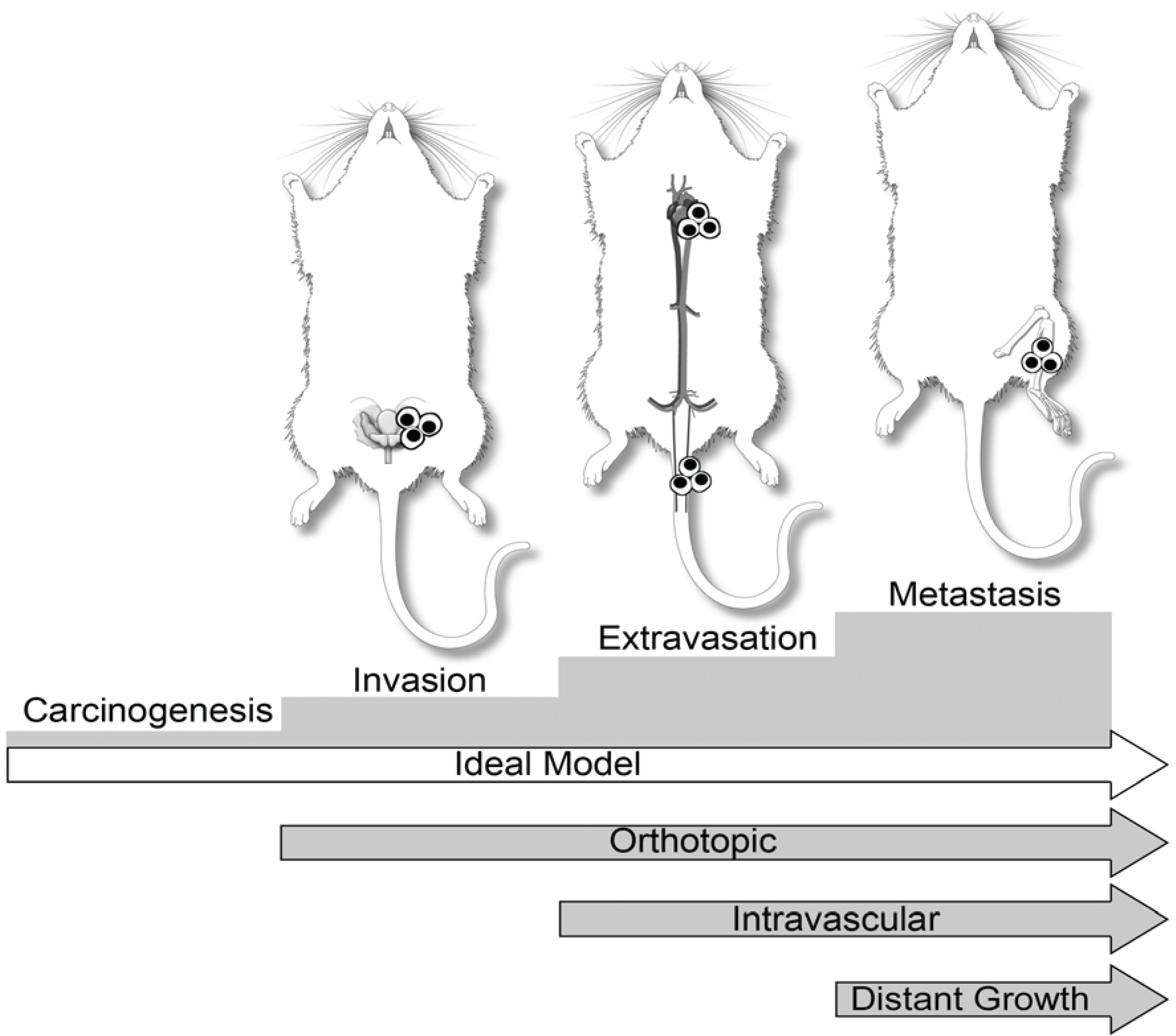
3. Dogs in Prostate Cancer Research
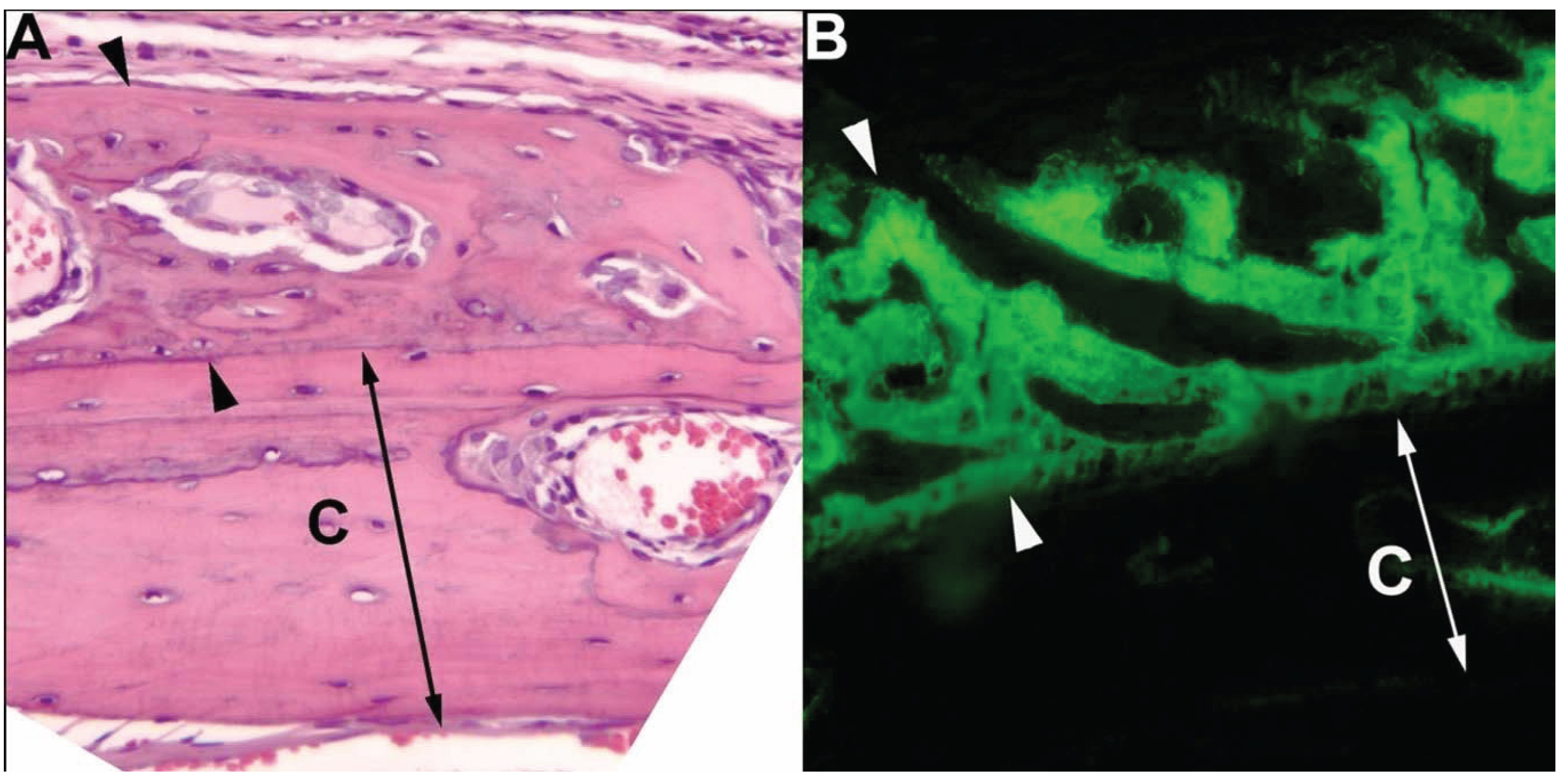
| Cell Line | Models | Notes |
|---|---|---|
| DCP-1 | Subcutaneous xenograft in mice | Mixed osteoblastic/osteolytic metastases to pelvic bones in allograft model |
| Ace-1 | Subcutaneous, intratibial, intracardiac, and intra-vossicle xenografts in mice | Mixed osteoblastic/osteolytic tumors in intratibial, intracardiac and intra-vossicle models. Metastasis to long bones, ribs and vertebrae in intracardiac model |
| Leo | Subcutaneous, intratibial and intracardiac xenografts in mice | Osteolytic tumors in intratibial and intracardiac models. Metastasis primarily to brain and spinal cord, but also long bones in intracardiac model |
| Probasco | Subcutaneous, intratibial and intracardiac xenografts in mice | Osteoblastic tumors in intratibial and intracardiac models. Metastasis primarily to long bones |
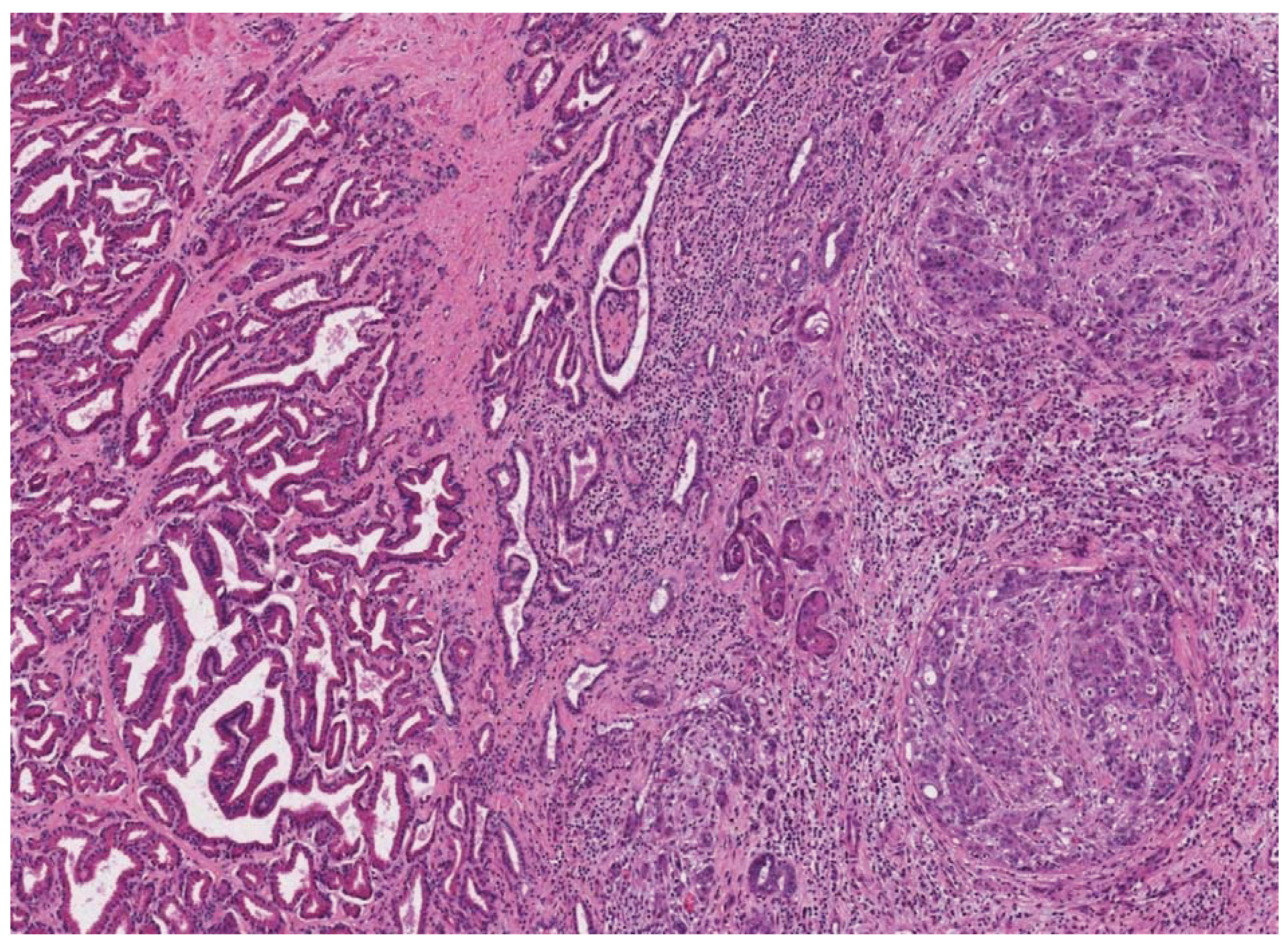
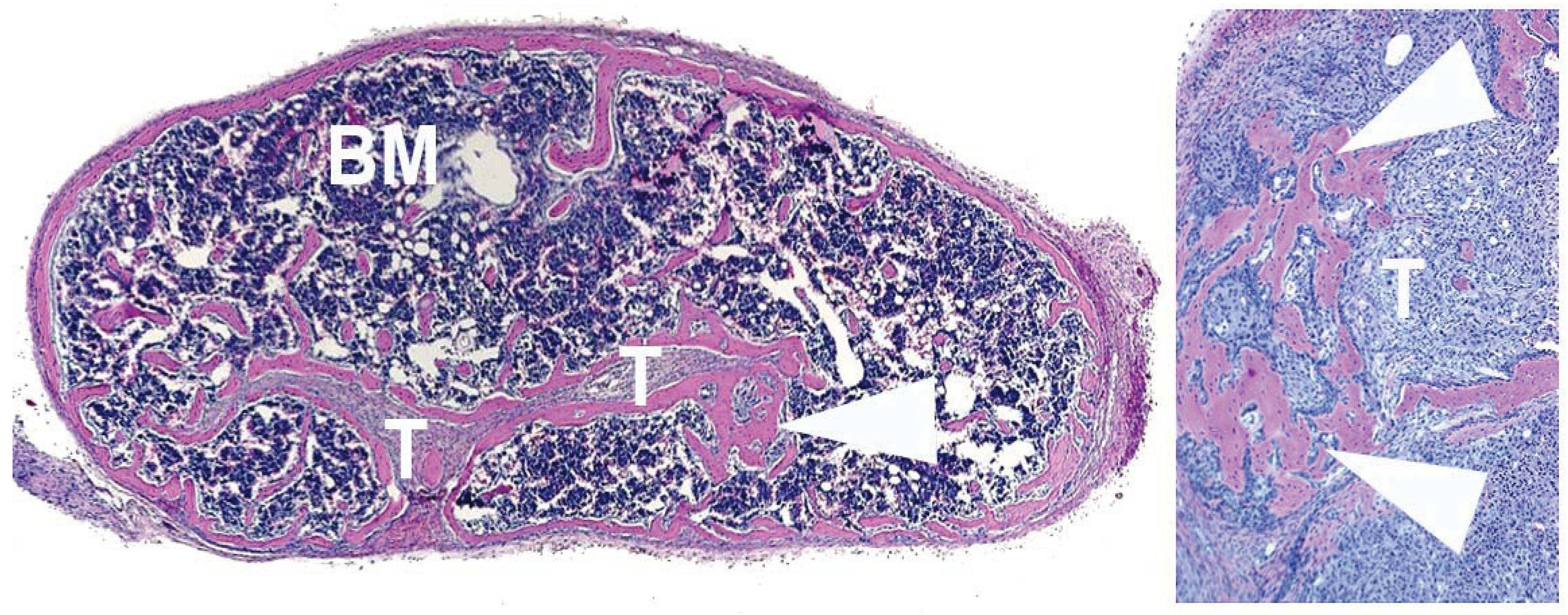

4. Human Prostate Cancer Xenografts in Immunocompromised Mice
| Cell line | Models | Notes |
|---|---|---|
| PC3 | Orthotopic, intratibial, and intracardiac injection | Osteolytic tumors Metastasizes to skull, ribs, pelvis, femur and tibia after orthotopic or intracardiac injection |
| PC3M | Orthotopic and intracardiac injection | Osteolytic tumors with metastasis primarily to mandible |
| LAPC-4 | Orthotopic injection | Mixed osteoblastic/osteolytic tumors |
| LAPC-9 | Intratibial injection | Osteoblastic tumors |
| LNCAP | Human adult bone with intra-bone or tail vain injection | Mixed osteoblastic/osteolytic tumors |
| LNCaP C4-2 | Subcutaneous, orthotopic, and intracardiac injection | Vertebral osteolytic metastases |
| LNCaP C4-2B4 | Intrafemoral | Mixed osteoblastic/osteolytic tumors |
| LNCaP CL-1 | Orthotopic injection | Osteolytic tumors |
| MDA PCa 2b | Intrafemoral injection | Osteoblastic tumors |
| CWR22R | Subcutaneous injection | LacZ-positive bone micro-metastases |
| ARCaP | Orthotopic injection | Mixed osteoblastic/osteolytic tumors |
| LuCap 35 | Intratibial injection Orthotopic injection | Osteolytic tumors in intratibial injections |
| LuCaP 23.1 | Intratibial injection | Osteoblastic tumors |
| Wish-pc2 | Intrafemoral or intratibial injection | Osteolytic tumors |
5. Prostate Cancer in Mice
6. Mouse Prostate Reconstitution Model
7. End-Stage Mouse Models of Tumor/Bone Interactions
8. Prostate Cancer in Rats
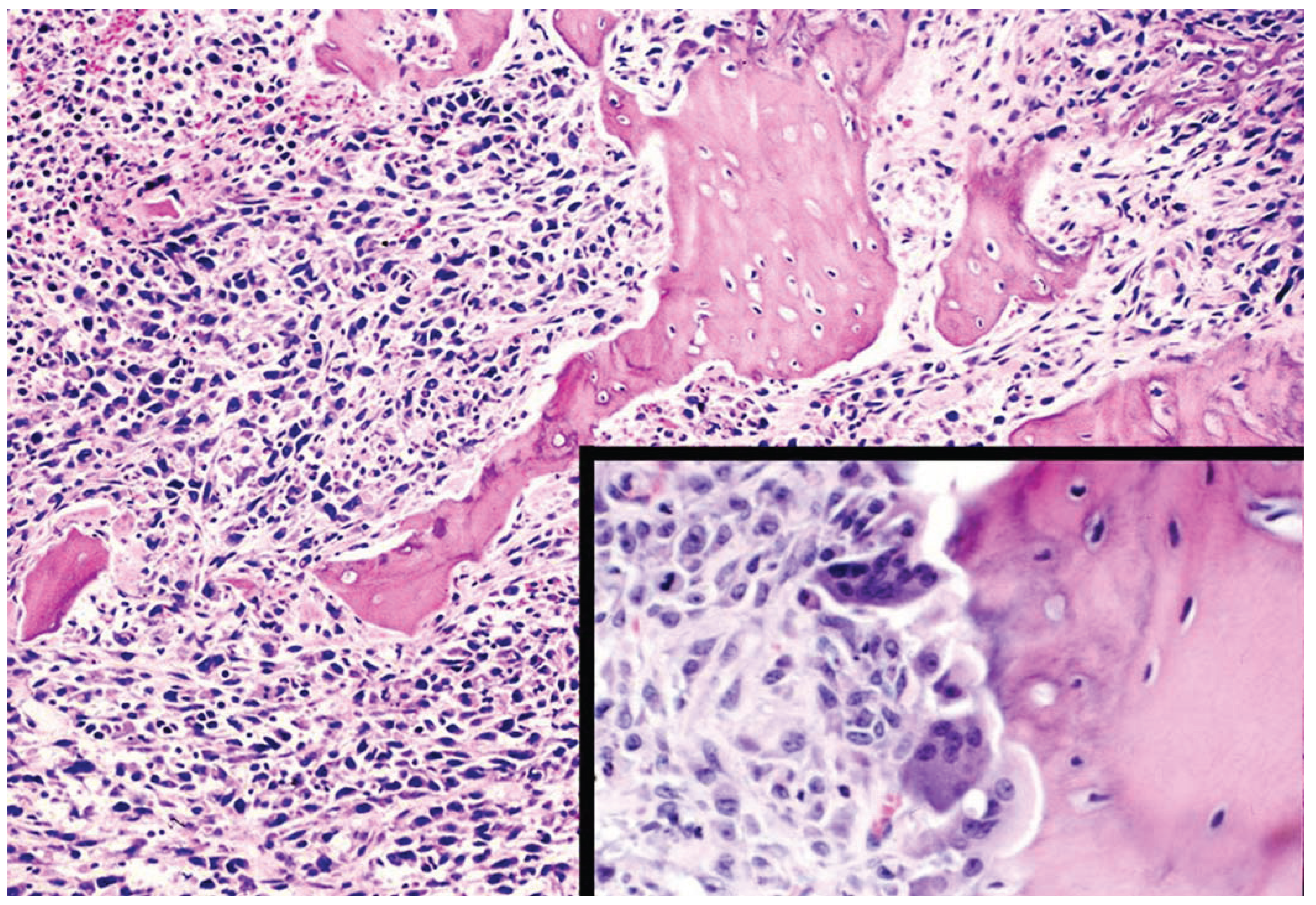
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA: Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef]
- Mehra, R.; Kumar-Sinha, C.; Shankar, S.; Lonigro, R.J.; Jing, X.; Philips, N.E.; Siddiqui, J.; Han, B.; Cao, X.; Smith, D.C.; et al. Characterization of bone metastases from rapid autopsies of prostate cancer patients. Clin. Cancer Res. 2011, 17, 3924–3932. [Google Scholar] [CrossRef]
- Sturge, J.; Caley, M.P.; Waxman, J. Bone metastasis in prostate cancer: Emerging therapeutic strategies. Nat. Rev. Clin. Oncol. 2011, 8, 357–368. [Google Scholar]
- Heidenreich, A. Bisphosphonates in the management of metastatic prostate cancer. Oncology 2003, 65, 5–11. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef]
- Morgans, A.K.; Smith, M.R. Bone-targeted agents: Preventing skeletal complications in prostate cancer. Urol. Clin. North Am. 2012, 39, 533–546. [Google Scholar] [CrossRef]
- Langdon, S.P. Animal modeling of cancer pathology and studying tumor response to therapy. Curr. Drug Targets 2012, 13, 1535–1547. [Google Scholar] [CrossRef]
- Waters, D.J.; Hayden, D.W.; Bell, F.W.; Klausner, J.S.; Qian, J.; Bostwick, D.G. Prostatic intraepithelial neoplasia in dogs with spontaneous prostate cancer. Prostate 1997, 30, 92–97. [Google Scholar] [CrossRef]
- Gamlem, H.; Nordstoga, K.; Glattre, E. Canine neoplasia—Introductory paper. APMIS. Suppl. 2008, 125, 5–18. [Google Scholar] [CrossRef]
- Rosol, T.J.; Tannehill-Gregg, S.H.; LeRoy, B.E.; Mandl, S.; Contag, C.H. Animal models of bone metastasis. Cancer 2003, 97, 748–757. [Google Scholar] [CrossRef]
- Smith, J. Canine prostatic disease: A review of anatomy, pathology, diagnosis, and treatment. Theriogenology 2008, 70, 375–383. [Google Scholar] [CrossRef]
- Waters, D.J.; Bostwick, D.G. Prostatic intraepithelial neoplasia occurs spontaneously in the canine prostate. J. Urol. 1997, 157, 713–716. [Google Scholar] [CrossRef]
- Madewell, B.R.; Gandour-Edwards, R.; DeVere White, R.W. Canine prostatic intraepithelial neoplasia: Is the comparative model relevant? Prostate 2004, 58, 314–317. [Google Scholar] [CrossRef]
- Teske, E.; Naan, E.C.; van Dijk, E.M.; van Garderen, E.; Schalken, J.A. Canine prostate carcinoma: Epidemiological evidence of an increased risk in castrated dogs. Mol. Cell. Endocrinol. 2002, 197, 251–255. [Google Scholar] [CrossRef]
- Moulay, M.; Liu, W.; Willenbrock, S.; Sterenczak, K.A.; Carlson, R.; Ngezahayo, A.; Murua Escobar, H.; Nolte, I. Evaluation of stem cell marker gene expression in canine prostate carcinoma- and prostate cyst-derived cell lines. Anticancer Res. 2013, 33, 5421–5431. [Google Scholar]
- Sharifi, N. Mechanisms of androgen receptor activation in castration-resistant prostate cancer. Endocrinology 2013, 154, 4010–4017. [Google Scholar] [CrossRef]
- Derleth, C.L.; Yu, E.Y. Targeted therapy in the treatment of castration-resistant prostate cancer. Oncology (Williston Park) 2013, 27, 620–628. [Google Scholar]
- Gallardo, F.; Mogas, T.; Baro, T.; Rabanal, R.; Morote, J.; Abal, M.; Reventos, J.; Lloreta, J. Expression of androgen, oestrogen alpha and beta, and progesterone receptors in the canine prostate: Differences between normal, inflamed, hyperplastic and neoplastic glands. J. Comp. Pathol. 2007, 136, 1–8. [Google Scholar] [CrossRef]
- Leav, I.; Schelling, K.H.; Adams, J.Y.; Merk, F.B.; Alroy, J. Role of canine basal cells in postnatal prostatic development, induction of hyperplasia, and sex hormone-stimulated growth; and the ductal origin of carcinoma. Prostate 2001, 48, 210–224. [Google Scholar] [CrossRef]
- Genega, E.M.; Hutchinson, B.; Reuter, V.E.; Gaudin, P.B. Immunophenotype of high-grade prostatic adenocarcinoma and urothelial carcinoma. Mod. Pathol. 2000, 13, 1186–1191. [Google Scholar] [CrossRef]
- Gobello, C.; Castex, G.; Corrada, Y. Serum and seminal markers in the diagnosis of disorders of the genital tract of the dog: A mini-review. Theriogenology 2002, 57, 1285–1291. [Google Scholar] [CrossRef]
- LeRoy, B.E.; Nadella, M.V.; Toribio, R.E.; Leav, I.; Rosol, T.J. Canine prostate carcinomas express markers of urothelial and prostatic differentiation. Vet. Pathol. 2004, 41, 131–140. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ricklis, R.M.; Williams, S.A.; Denmeade, S.R. Comparative study of psma expression in the prostate of mouse, dog, monkey, and human. Prostate 2006, 66, 903–910. [Google Scholar] [CrossRef]
- Wu, L.Y.; Johnson, J.M.; Simmons, J.K.; Mendes, D.E.; Geruntho, J.J.; Liu, T.; Dirksen, W.P.; Rosol, T.J.; Davis, W.C.; Berkman, C.E. Biochemical characterization of prostate-specific membrane antigen from canine prostate carcinoma cells. Prostate 2014, 74, 451–457. [Google Scholar] [CrossRef]
- Paner, G.P.; Luthringer, D.J.; Amin, M.B. Best practice in diagnostic immunohistochemistry: Prostate carcinoma and its mimics in needle core biopsies. Arch. Pathol. Lab. Med. 2008, 132, 1388–1396. [Google Scholar]
- McEntee, M.; Isaacs, W.; Smith, C. Adenocarcinoma of the canine prostate: Immunohistochemical examination for secretory antigens. Prostate 1987, 11, 163–170. [Google Scholar] [CrossRef]
- LeRoy, B.; Painter, A.; Sheppard, H.; Popiolek, L.; Samuel-Foo, M.; Andacht, T.M. Protein expression profiling of normal and neoplastic canine prostate and bladder tissue. Vet. Comp. Oncol. 2007, 5, 119–130. [Google Scholar] [CrossRef]
- Chen, Y.; Scher, H.I. Prostate cancer in 2011: Hitting old targets better and identifying new targets. Nat. Rev. Clin. Oncol. 2012, 9, 70–72. [Google Scholar] [CrossRef]
- Meuten, D.J. Tumors in Domestic Animals, 4th ed.; Wiley-Blackwell: Ames, IA, USA, 2002. [Google Scholar]
- Cornell, K.K.; Bostwick, D.G.; Cooley, D.M.; Hall, G.; Harvey, H.J.; Hendrick, M.J.; Pauli, B.U.; Render, J.A.; Stoica, G.; Sweet, D.C.; et al. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: A retrospective analysis of 76 cases. Prostate 2000, 45, 173–183. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; Williams, B.O. Mouse models of prostate cancer. Prostate Cancer 2011, 2011. [Google Scholar] [CrossRef]
- LeRoy, B.E.; Bahnson, R.R.; Rosol, T.J. New bone formation in nude mouse calvaria induced by canine prostate tissue. Mol. Cell. Endocrinol. 2002, 197, 257–263. [Google Scholar] [CrossRef]
- LeRoy, B.E.; Sellers, R.S.; Rosol, T.J. Canine prostate stimulates osteoblast function using the endothelin receptors. Prostate 2004, 59, 148–156. [Google Scholar] [CrossRef]
- Heidegger, I.; Massoner, P.; Eder, I.E.; Pircher, A.; Pichler, R.; Aigner, F.; Bektic, J.; Horninger, W.; Klocker, H. Novel therapeutic approaches for the treatment of castration-resistant prostate cancer. J. Steroid Biochem. Mol. Biol. 2013, 138, 248–256. [Google Scholar] [CrossRef]
- Rove, K.O.; Crawford, E.D. Evolution of treatment options for patients with CRPC and bone metastases: Bone-targeted agents that go beyond palliation of symptoms to improve overall survival. Oncology (Williston Park) 2011, 25, 1362–1370, 1375–1381, 1387. [Google Scholar]
- Pinto, A.; Merino, M.; Zamora, P.; Redondo, A.; Castelo, B.; Espinosa, E. Targeting the endothelin axis in prostate carcinoma. Tumour Biol. 2012, 33, 421–426. [Google Scholar] [CrossRef]
- Roh, M.; Abdulkadir, S.A. Targeting the endothelin receptor in prostate cancer bone metastasis: Back to the mouse? Cancer Biol. Ther. 2010, 9, 615–617. [Google Scholar]
- Shao, N.; Wang, Y.; Jiang, W.Y.; Qiao, D.; Zhang, S.G.; Wu, Y.; Zhang, X.X.; Wang, J.L.; Ding, Y.; Feng, N.H. Immunotherapy and endothelin receptor antagonists for treatment of castration-resistant prostate cancer. Int. J. Cancer. 2013, 133, 1743–1750. [Google Scholar] [CrossRef]
- Ishizaka, K.; Azuma, H.; Matsubara, O.; Kitahara, S.; Oshima, H. Production of endothelin by canine prostatic epithelial cells and its stimulatory effects on their growth. J. Androl. 1999, 20, 529–536. [Google Scholar]
- Blomme, E.A.; Sugimoto, Y.; McCauley, L.K.; Lin, Y.C.; Capen, C.C.; Rosol, T.J. Stromal and epithelial cells of the canine prostate express parathyroid hormone-related protein, but not the pth/pthrp receptor. Prostate 1998, 36, 110–120. [Google Scholar] [CrossRef]
- Anidjar, M.; Villette, J.M.; Devauchelle, P.; Delisle, F.; Cotard, J.P.; Billotey, C.; Cochand-Priollet, B.; Copin, H.; Barnoux, M.; Triballeau, S.; et al. In vivo model mimicking natural history of dog prostate cancer using DPC-1, a new canine prostate carcinoma cell line. Prostate 2001, 46, 2–10. [Google Scholar] [CrossRef]
- Eaton, C.L.; Pierrepoint, C.G. Growth of a spontaneous canine prostatic adenocarcinoma in vivo and in vitro: Isolation and characterization of a neoplastic prostatic epithelial cell line, CPA 1. Prostate 1988, 12, 129–143. [Google Scholar] [CrossRef]
- LeRoy, B.E.; Thudi, N.K.; Nadella, M.V.; Toribio, R.E.; Tannehill-Gregg, S.H.; van Bokhoven, A.; Davis, D.; Corn, S.; Rosol, T.J. New bone formation and osteolysis by a metastatic, highly invasive canine prostate carcinoma xenograft. Prostate 2006, 66, 1213–1222. [Google Scholar] [CrossRef]
- Thudi, N.K.; Shu, S.T.; Martin, C.K.; Lanigan, L.G.; Nadella, M.V.; Van Bokhoven, A.; Werbeck, J.L.; Simmons, J.K.; Murahari, S.; Kisseberth, W.C.; et al. Development of a brain metastatic canine prostate cancer cell line. Prostate 2011, 71, 1251–1263. [Google Scholar]
- Fork, M.A.; Murua Escobar, H.; Soller, J.T.; Sterenczak, K.A.; Willenbrock, S.; Winkler, S.; Dorsch, M.; Reimann-Berg, N.; Hedrich, H.J.; Bullerdiek, J.; et al. Establishing an in vivo model of canine prostate carcinoma using the new cell line ct1258. BMC Cancer 2008, 8. [Google Scholar] [CrossRef]
- Anidjar, M.; Scarlata, E.; Cury, F.L.; Rocha, J.; Hamel, L.; Luz, M.; Chevalier, S. Refining the orthotopic dog prostate cancer (DPC)-1 model to better bridge the gap between rodents and men. Prostate 2012, 72, 752–761. [Google Scholar] [CrossRef]
- Li, X.; Liao, J.; Park, S.I.; Koh, A.J.; Sadler, W.D.; Pienta, K.J.; Rosol, T.J.; McCauley, L.K. Drugs which inhibit osteoclast function suppress tumor growth through calcium reduction in bone. Bone 2011, 48, 1354–1361. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Koh, A.J.; Berry, J.E.; Thudi, N.; Rosol, T.J.; Pienta, K.J.; McCauley, L.K. Tumor expressed pthrp facilitates prostate cancer-induced osteoblastic lesions. Int. J. Cancer. 2008, 123, 2267–2278. [Google Scholar] [CrossRef]
- Wise-Milestone, L.; Akens, M.K.; Rosol, T.J.; Hojjat, S.P.; Grynpas, M.D.; Whyne, C.M. Evaluating the effects of mixed osteolytic/osteoblastic metastasis on vertebral bone quality in a new rat model. J. Orthop. Res. 2012, 30, 817–823. [Google Scholar] [CrossRef]
- Schade, G.R.; Keller, J.; Ives, K.; Cheng, X.; Rosol, T.J.; Keller, E.; Roberts, W.W. Histotripsy focal ablation of implanted prostate tumor in an ACE-1 canine cancer model. J. Urol. 2012, 188, 1957–1964. [Google Scholar] [CrossRef]
- Halvorson, K.G.; Kubota, K.; Sevcik, M.A.; Lindsay, T.H.; Sotillo, J.E.; Ghilardi, J.R.; Rosol, T.J.; Boustany, L.; Shelton, D.L.; Mantyh, P.W. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005, 65, 9426–9435. [Google Scholar] [CrossRef]
- Halvorson, K.G.; Sevcik, M.A.; Ghilardi, J.R.; Rosol, T.J.; Mantyh, P.W. Similarities and differences in tumor growth, skeletal remodeling and pain in an osteolytic and osteoblastic model of bone cancer. Clin. J. Pain 2006, 22, 587–600. [Google Scholar] [CrossRef]
- Thudi, N.K.; Martin, C.K.; Murahari, S.; Shu, S.T.; Lanigan, L.G.; Werbeck, J.L.; Keller, E.T.; McCauley, L.K.; Pinzone, J.J.; Rosol, T.J. Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate 2011, 71, 615–625. [Google Scholar] [CrossRef]
- Simmons, J.K.; Dirksen, W.P.; Hildreth, B.E., 3rd; Dorr, C.; Williams, C.; Thomas, R.; Breen, M.; Toribio, R.E.; Rosol, T.J. Canine prostate cancer cell line (probasco) produces osteoblastic metastases in vivo. Prostate 2014, PROS-14-178, in press. [Google Scholar]
- Navone, N.M.; Logothetis, C.J.; von Eschenbach, A.C.; Troncoso, P. Model systems of prostate cancer: Uses and limitations. Cancer Metastasis Rev. 1998, 17, 361–371. [Google Scholar] [CrossRef]
- Keller, J.M.; Schade, G.R.; Ives, K.; Cheng, X.; Rosol, T.J.; Piert, M.; Siddiqui, J.; Roberts, W.W.; Keller, E.T. A novel canine model for prostate cancer. Prostate 2013, 73, 952–959. [Google Scholar] [CrossRef]
- Lee, Y.P.; Schwarz, E.M.; Davies, M.; Jo, M.; Gates, J.; Zhang, X.; Wu, J.; Lieberman, J.R. Use of zoledronate to treat osteoblastic versus osteolytic lesions in a severe-combined-immunodeficient mouse model. Cancer Res. 2002, 62, 5564–5570. [Google Scholar]
- Wu, T.T.; Sikes, R.A.; Cui, Q.; Thalmann, G.N.; Kao, C.; Murphy, C.F.; Yang, H.; Zhau, H.E.; Balian, G.; Chung, L.W. Establishing human prostate cancer cell xenografts in bone: Induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int. J. Cancer 1998, 77, 887–894. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, P.; Sun, F.X.; Hasegawa, S.; Baranov, E.; Chishima, T.; Shimada, H.; Moossa, A.R.; Hoffman, R.M. A fluorescent orthotopic bone metastasis model of human prostate cancer. Cancer Res. 1999, 59, 781–786. [Google Scholar]
- Nemeth, J.A.; Harb, J.F.; Barroso, U., Jr.; He, Z.; Grignon, D.J.; Cher, M.L. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res. 1999, 59, 1987–1993. [Google Scholar]
- Yonou, H.; Yokose, T.; Kamijo, T.; Kanomata, N.; Hasebe, T.; Nagai, K.; Hatano, T.; Ogawa, Y.; Ochiai, A. Establishment of a novel species- and tissue-specific metastasis model of human prostate cancer in humanized non-obese diabetic/severe combined immunodeficient mice engrafted with human adult lung and bone. Cancer Res. 2001, 61, 2177–2182. [Google Scholar]
- Pettaway, C.A.; Pathak, S.; Greene, G.; Ramirez, E.; Wilson, M.R.; Killion, J.J.; Fidler, I.J. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin. Cancer Res. 1996, 2, 1627–1636. [Google Scholar]
- Kozlowski, J.M.; Fidler, I.J.; Campbell, D.; Xu, Z.L.; Kaighn, M.E.; Hart, I.R. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984, 44, 3522–3529. [Google Scholar]
- Parkins, G.E.; Klufio, G.O. Prostate cancer metastasis to the mandible: Case report. East Afr. Med. J. 2009, 86, 251–252. [Google Scholar]
- Craft, N.; Chhor, C.; Tran, C.; Belldegrun, A.; DeKernion, J.; Witte, O.N.; Said, J.; Reiter, R.E.; Sawyers, C.L. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 1999, 59, 5030–5036. [Google Scholar]
- Davies, M.R.; Lee, Y.P.; Lee, C.; Zhang, X.; Afar, D.E.; Lieberman, J.R. Use of a scid mouse model to select for a more aggressive strain of prostate cancer. Anticancer Res. 2003, 23, 2245–2252. [Google Scholar]
- Tsingotjidou, A.S.; Zotalis, G.; Jackson, K.R.; Sawyers, C.; Puzas, J.E.; Hicks, D.G.; Reiter, R.; Lieberman, J.R. Development of an animal model for prostate cancer cell metastasis to adult human bone. Anticancer Res. 2001, 21, 971–978. [Google Scholar]
- Yonou, H.; Kanomata, N.; Goya, M.; Kamijo, T.; Yokose, T.; Hasebe, T.; Nagai, K.; Hatano, T.; Ogawa, Y.; Ochiai, A. Osteoprotegerin/osteoclastogenesis inhibitory factor decreases human prostate cancer burden in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice. Cancer Res. 2003, 63, 2096–2102. [Google Scholar]
- Dias, S.J.; Li, K.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Levenson, A.S. Trimethoxy-resveratrol and piceatannol administered orally suppress and inhibit tumor formation and growth in prostate cancer xenografts. Prostate 2013, 73, 1135–1146. [Google Scholar] [CrossRef]
- Scatena, C.D.; Hepner, M.A.; Oei, Y.A.; Dusich, J.M.; Yu, S.F.; Purchio, T.; Contag, P.R.; Jenkins, D.E. Imaging of bioluminescent LNCaP-luc-M6 tumors: A new animal model for the study of metastatic human prostate cancer. Prostate 2004, 59, 292–303. [Google Scholar] [CrossRef]
- Thalmann, G.N.; Anezinis, P.E.; Chang, S.M.; Zhau, H.E.; Kim, E.E.; Hopwood, V.L.; Pathak, S.; von Eschenbach, A.C.; Chung, L.W. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994, 54, 2577–2581. [Google Scholar]
- Tso, C.L.; McBride, W.H.; Sun, J.; Patel, B.; Tsui, K.H.; Paik, S.H.; Gitlitz, B.; Caliliw, R.; van Ophoven, A.; Wu, L.; et al. Androgen deprivation induces selective outgrowth of aggressive hormone-refractory prostate cancer clones expressing distinct cellular and molecular properties not present in parental androgen-dependent cancer cells. Cancer J. 2000, 6, 220–233. [Google Scholar]
- Navone, N.M.; Olive, M.; Ozen, M.; Davis, R.; Troncoso, P.; Tu, S.M.; Johnston, D.; Pollack, A.; Pathak, S.; von Eschenbach, A.C.; et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin. Cancer Res. 1997, 3, 2493–2500. [Google Scholar]
- Yang, J.; Fizazi, K.; Peleg, S.; Sikes, C.R.; Raymond, A.K.; Jamal, N.; Hu, M.; Olive, M.; Martinez, L.A.; Wood, C.G.; et al. Prostate cancer cells induce osteoblast differentiation through a cbfa1-dependent pathway. Cancer Res. 2001, 61, 5652–5659. [Google Scholar]
- Goya, M.; Miyamoto, S.; Nagai, K.; Ohki, Y.; Nakamura, K.; Shitara, K.; Maeda, H.; Sangai, T.; Kodama, K.; Endoh, Y.; et al. Growth inhibition of human prostate cancer cells in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice by a ligand-specific antibody to human insulin-like growth factors. Cancer Res. 2004, 64, 6252–6258. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Malloy, P.J.; Krishnan, A.V.; Swami, S.; Navone, N.M.; Peehl, D.M.; Feldman, D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat. Med. 2000, 6, 703–706. [Google Scholar] [CrossRef]
- Hara, T.; Nakamura, K.; Araki, H.; Kusaka, M.; Yamaoka, M. Enhanced androgen receptor signaling correlates with the androgen-refractory growth in a newly established MDA PCa 2b-hr human prostate cancer cell subline. Cancer Res. 2003, 63, 5622–5628. [Google Scholar]
- Zhao, X.Y.; Boyle, B.; Krishnan, A.V.; Navone, N.M.; Peehl, D.M.; Feldman, D. Two mutations identified in the androgen receptor of the new human prostate cancer cell line MDA PCa 2a. J. Urol. 1999, 162, 2192–2199. [Google Scholar] [CrossRef]
- Liu, X.H.; Kirschenbaum, A.; Yao, S.; Liu, G.; Aaronson, S.A.; Levine, A.C. Androgen-induced wnt signaling in preosteoblasts promotes the growth of MDA-PCa-2b human prostate cancer cells. Cancer Res. 2007, 67, 5747–5753. [Google Scholar] [CrossRef]
- Pretlow, T.G.; Wolman, S.R.; Micale, M.A.; Pelley, R.J.; Kursh, E.D.; Resnick, M.I.; Bodner, D.R.; Jacobberger, J.W.; Delmoro, C.M.; Giaconia, J.M.; et al. Xenografts of primary human prostatic carcinoma. J. Natl. Cancer Inst. 1993, 85, 394–398. [Google Scholar] [CrossRef]
- Holleran, J.L.; Miller, C.J.; Culp, L.A. Tracking micrometastasis to multiple organs with lacZ-tagged CWR22R prostate carcinoma cells. J. Histochem. Cytochem. 2000, 48, 643–651. [Google Scholar] [CrossRef]
- Zhau, H.Y.; Chang, S.M.; Chen, B.Q.; Wang, Y.; Zhang, H.; Kao, C.; Sang, Q.A.; Pathak, S.J.; Chung, L.W. Androgen-repressed phenotype in human prostate cancer. Proc. Natl. Acad. Sci. U.S.A 1996, 93, 15152–15157. [Google Scholar] [CrossRef]
- Corey, E.; Quinn, J.E.; Buhler, K.R.; Nelson, P.S.; Macoska, J.A.; True, L.D.; Vessella, R.L. LuCaP 35: A new model of prostate cancer progression to androgen independence. Prostate 2003, 55, 239–246. [Google Scholar] [CrossRef]
- Ellis, W.J.; Vessella, R.L.; Buhler, K.R.; Bladou, F.; True, L.D.; Bigler, S.A.; Curtis, D.; Lange, P.H. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clin. Cancer Res. 1996, 2, 1039–1048. [Google Scholar]
- Corey, E.; Quinn, J.E.; Bladou, F.; Brown, L.G.; Roudier, M.P.; Brown, J.M.; Buhler, K.R.; Vessella, R.L. Establishment and characterization of osseous prostate cancer models: Intra-tibial injection of human prostate cancer cells. Prostate 2002, 52, 20–33. [Google Scholar] [CrossRef]
- Corey, E.; Quinn, J.E.; Vessella, R.L. A novel method of generating prostate cancer metastases from orthotopic implants. Prostate 2003, 56, 110–114. [Google Scholar] [CrossRef]
- Pinthus, J.H.; Waks, T.; Schindler, D.G.; Harmelin, A.; Said, J.W.; Belldegrun, A.; Ramon, J.; Eshhar, Z. WISH-PC2: A unique xenograft model of human prostatic small cell carcinoma. Cancer Res. 2000, 60, 6563–6567. [Google Scholar]
- Lee, C.H.; Akin-Olugbade, O.; Kirschenbaum, A. Overview of prostate anatomy, histology, and pathology. Endocrinol. Metab. Clin. North Am. 2011, 40, 565–575. [Google Scholar] [CrossRef]
- Shappell, S.B.; Thomas, G.V.; Roberts, R.L.; Herbert, R.; Ittmann, M.M.; Rubin, M.A.; Humphrey, P.A.; Sundberg, J.P.; Rozengurt, N.; Barrios, R.; et al. Prostate pathology of genetically engineered mice: Definitions and classification. The consensus report from the bar harbor meeting of the mouse models of human cancer consortium prostate pathology committee. Cancer Res. 2004, 64, 2270–2305. [Google Scholar] [CrossRef]
- Kasper, S. Survey of genetically engineered mouse models for prostate cancer: Analyzing the molecular basis of prostate cancer development, progression, and metastasis. J. Cell. Biochem. 2005, 94, 279–297. [Google Scholar] [CrossRef]
- Berman-Booty, L.D.; Sargeant, A.M.; Rosol, T.J.; Rengel, R.C.; Clinton, S.K.; Chen, C.S.; Kulp, S.K. A review of the existing grading schemes and a proposal for a modified grading scheme for prostatic lesions in tramp mice. Toxicol. Pathol. 2012, 40, 5–17. [Google Scholar] [CrossRef]
- Berman-Booty, L.D.; Chu, P.C.; Thomas-Ahner, J.M.; Bolon, B.; Wang, D.; Yang, T.; Clinton, S.K.; Kulp, S.K.; Chen, C.S. Suppression of prostate epithelial proliferation and intraprostatic progrowth signaling in transgenic mice by a new energy restriction-mimetic agent. Cancer Prev. Res. 2013, 6, 232–241. [Google Scholar] [CrossRef]
- Sargeant, A.M.; Klein, R.D.; Rengel, R.C.; Clinton, S.K.; Kulp, S.K.; Kashida, Y.; Yamaguchi, M.; Wang, X.; Chen, C.S. Chemopreventive and bioenergetic signaling effects of PDK1/Akt pathway inhibition in a transgenic mouse model of prostate cancer. Toxicol. Pathol. 2007, 35, 549–561. [Google Scholar] [CrossRef]
- Patel, S.J.; Molinolo, A.A.; Gutkind, S.; Crawford, N.P. Germline genetic variation modulates tumor progression and metastasis in a mouse model of neuroendocrine prostate carcinoma. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Gingrich, J.R.; Barrios, R.J.; Morton, R.A.; Boyce, B.F.; DeMayo, F.J.; Finegold, M.J.; Angelopoulou, R.; Rosen, J.M.; Greenberg, N.M. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996, 56, 4096–4102. [Google Scholar]
- Hensley, P.J.; Kyprianou, N. Modeling prostate cancer in mice: Limitations and opportunities. J. Androl. 2012, 33, 133–144. [Google Scholar] [CrossRef]
- Roy-Burman, P.; Wu, H.; Powell, W.C.; Hagenkord, J.; Cohen, M.B. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr.-Relat. Cancer 2004, 11, 225–254. [Google Scholar] [CrossRef]
- Gupta, S.; Hastak, K.; Ahmad, N.; Lewin, J.S.; Mukhtar, H. Inhibition of prostate carcinogenesis in tramp mice by oral infusion of green tea polyphenols. Proc. Natl. Acad. Sci. USA 2001, 98, 10350–10355. [Google Scholar] [CrossRef]
- Klezovitch, O.; Chevillet, J.; Mirosevich, J.; Roberts, R.L.; Matusik, R.J.; Vasioukhin, V. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell 2004, 6, 185–195. [Google Scholar]
- Garabedian, E.M.; Humphrey, P.A.; Gordon, J.I. A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15382–15387. [Google Scholar] [CrossRef]
- Tu, W.H.; Thomas, T.Z.; Masumori, N.; Bhowmick, N.A.; Gorska, A.E.; Shyr, Y.; Kasper, S.; Case, T.; Roberts, R.L.; Shappell, S.B.; et al. The loss of tgf-beta signaling promotes prostate cancer metastasis. Neoplasia 2003, 5, 267–277. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, C.J.; Jaskelioff, M.; Ivanova, E.; Kost-Alimova, M.; Protopopov, A.; Chu, G.C.; Wang, G.; Lu, X.; Labrot, E.S.; et al. Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell 2012, 148, 896–907. [Google Scholar] [CrossRef]
- Wang, S.; Gao, J.; Lei, Q.; Rozengurt, N.; Pritchard, C.; Jiao, J.; Thomas, G.V.; Li, G.; Roy-Burman, P.; Nelson, P.S.; et al. Prostate-specific deletion of the murine pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 2003, 4, 209–221. [Google Scholar] [CrossRef]
- Perez-Stable, C.; Altman, N.H.; Mehta, P.P.; Deftos, L.J.; Roos, B.A. Prostate cancer progression, metastasis, and gene expression in transgenic mice. Cancer Res. 1997, 57, 900–906. [Google Scholar]
- Cai, Y.; Kregel, S.; Vander Griend, D.J. Formation of human prostate epithelium using tissue recombination of rodent urogenital sinus mesenchyme and human stem cells. J. Vis. Exp. 2013, 22. [Google Scholar] [CrossRef]
- Buttyan, R. The mouse prostate reconstitution model of prostate diseases. Prostate 1997, 33, 164–165. [Google Scholar] [CrossRef]
- Thompson, T.C.; Timme, T.L.; Park, S.H.; Yang, G.; Ren, C. Mouse prostate reconstitution model system: A series of in vivo and in vitro models for benign and malignant prostatic disease. Prostate 2000, 43, 248–254. [Google Scholar] [CrossRef]
- Cunha, G.R.; Hayward, S.W.; Wang, Y.Z. Role of stroma in carcinogenesis of the prostate. Differentiation 2002, 70, 473–485. [Google Scholar] [CrossRef]
- Thompson, T.C.; Park, S.H.; Timme, T.L.; Ren, C.; Eastham, J.A.; Donehower, L.A.; Bradley, A.; Kadmon, D.; Yang, G. Loss of p53 function leads to metastasis in ras + myc-initiated mouse prostate cancer. Oncogene 1995, 10, 869–879. [Google Scholar]
- Park, S.I.; Kim, S.J.; McCauley, L.K.; Gallick, G.E. Pre-clinical mouse models of human prostate cancer and their utility in drug discovery. Curr. Protoc. Pharmacol. 2010. [Google Scholar] [CrossRef]
- Blouin, S.; Basle, M.F.; Chappard, D. Rat models of bone metastases. Clin. Exp. Metastasis 2005, 22, 605–614. [Google Scholar] [CrossRef]
- Pollard, M.; Suckow, M.A. Hormone-refractory prostate cancer in the lobund-wistar rat. Exp. Biol. Med. 2005, 230, 520–526. [Google Scholar]
- Liepe, K.; Geidel, H.; Haase, M.; Hakenberg, O.W.; Runge, R.; Kotzerke, J. New model for the induction of osteoblastic bone metastases in rat. Anticancer Res. 2005, 25, 1067–1073. [Google Scholar]
- Geldof, A.A.; Rao, B.R. Prostatic tumor (r3327) skeletal metastasis. Prostate 1990, 16, 279–290. [Google Scholar] [CrossRef]
- Haq, M.; Goltzman, D.; Tremblay, G.; Brodt, P. Rat prostate adenocarcinoma cells disseminate to bone and adhere preferentially to bone marrow-derived endothelial cells. Cancer Res. 1992, 52, 4613–4619. [Google Scholar]
- Blomme, E.A.; Dougherty, K.M.; Pienta, K.J.; Capen, C.C.; Rosol, T.J.; McCauley, L.K. Skeletal metastasis of prostate adenocarcinoma in rats: Morphometric analysis and role of parathyroid hormone-related protein. Prostate 1999, 39, 187–197. [Google Scholar] [CrossRef]
- Tennant, T.R.; Kim, H.; Sokoloff, M.; Rinker-Schaeffer, C.W. The dunning model. Prostate 2000, 43, 295–302. [Google Scholar] [CrossRef]
- Koutsilieris, M. PA-III rat prostate adenocarcinoma cells (review). In Vivo 1992, 6, 199–203. [Google Scholar]
- Lorente, D.; de Bono, J.S. Molecular alterations and emerging targets in castration resistant prostate cancer. Eur. J. Cancer 2014, 50, 753–764. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Simmons, J.K.; Elshafae, S.M.; Keller, E.T.; McCauley, L.K.; Rosol, T.J. Review of Animal Models of Prostate Cancer Bone Metastasis. Vet. Sci. 2014, 1, 16-39. https://doi.org/10.3390/vetsci1010016
Simmons JK, Elshafae SM, Keller ET, McCauley LK, Rosol TJ. Review of Animal Models of Prostate Cancer Bone Metastasis. Veterinary Sciences. 2014; 1(1):16-39. https://doi.org/10.3390/vetsci1010016
Chicago/Turabian StyleSimmons, Jessica K., Said M. Elshafae, Evan T. Keller, Laurie K. McCauley, and Thomas J. Rosol. 2014. "Review of Animal Models of Prostate Cancer Bone Metastasis" Veterinary Sciences 1, no. 1: 16-39. https://doi.org/10.3390/vetsci1010016





