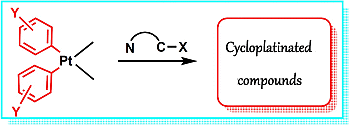Diarylplatinum(II) Compounds as Versatile Metallating Agents in the Synthesis of Cyclometallated Platinum Compounds with N-Donor Ligands
Abstract
:1. Introduction

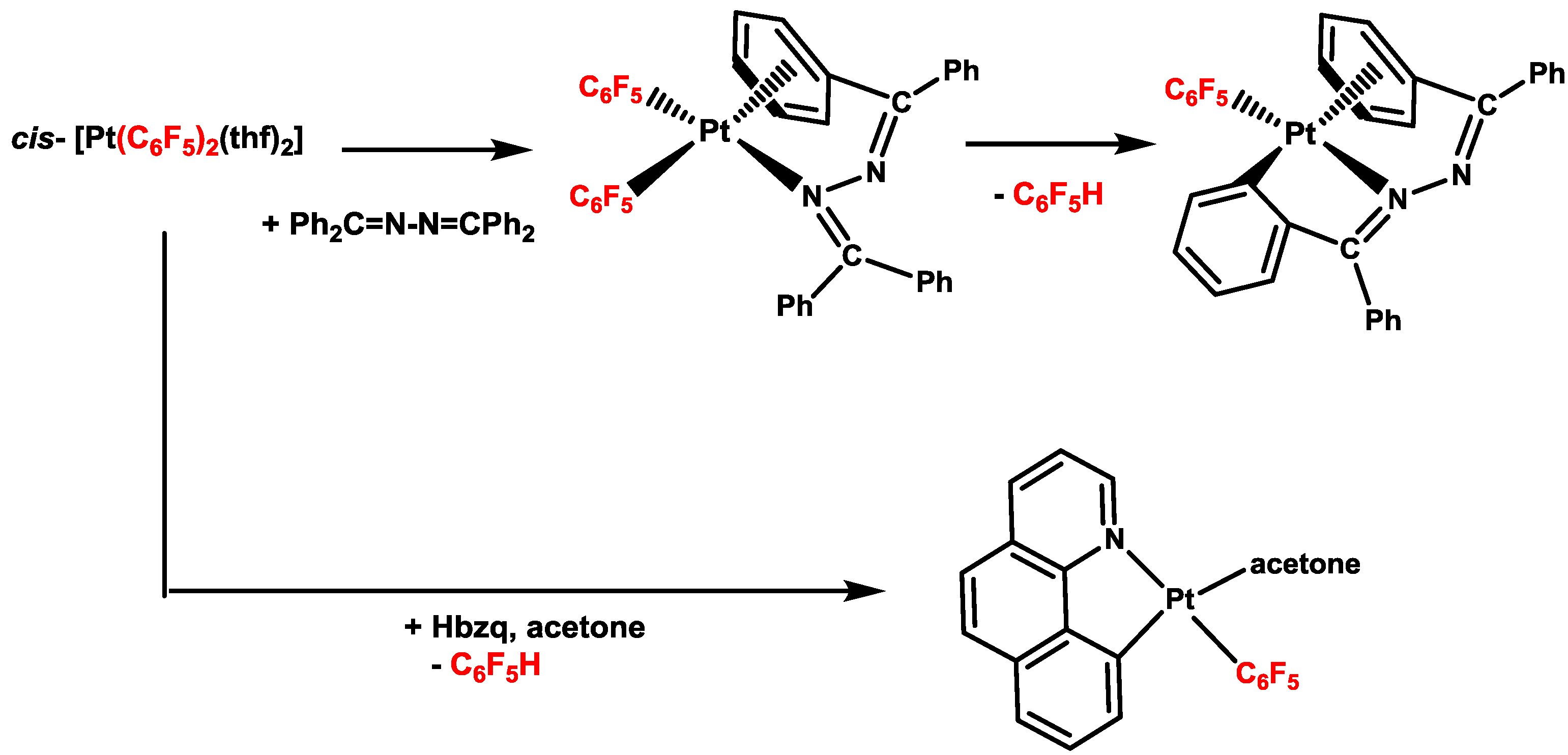
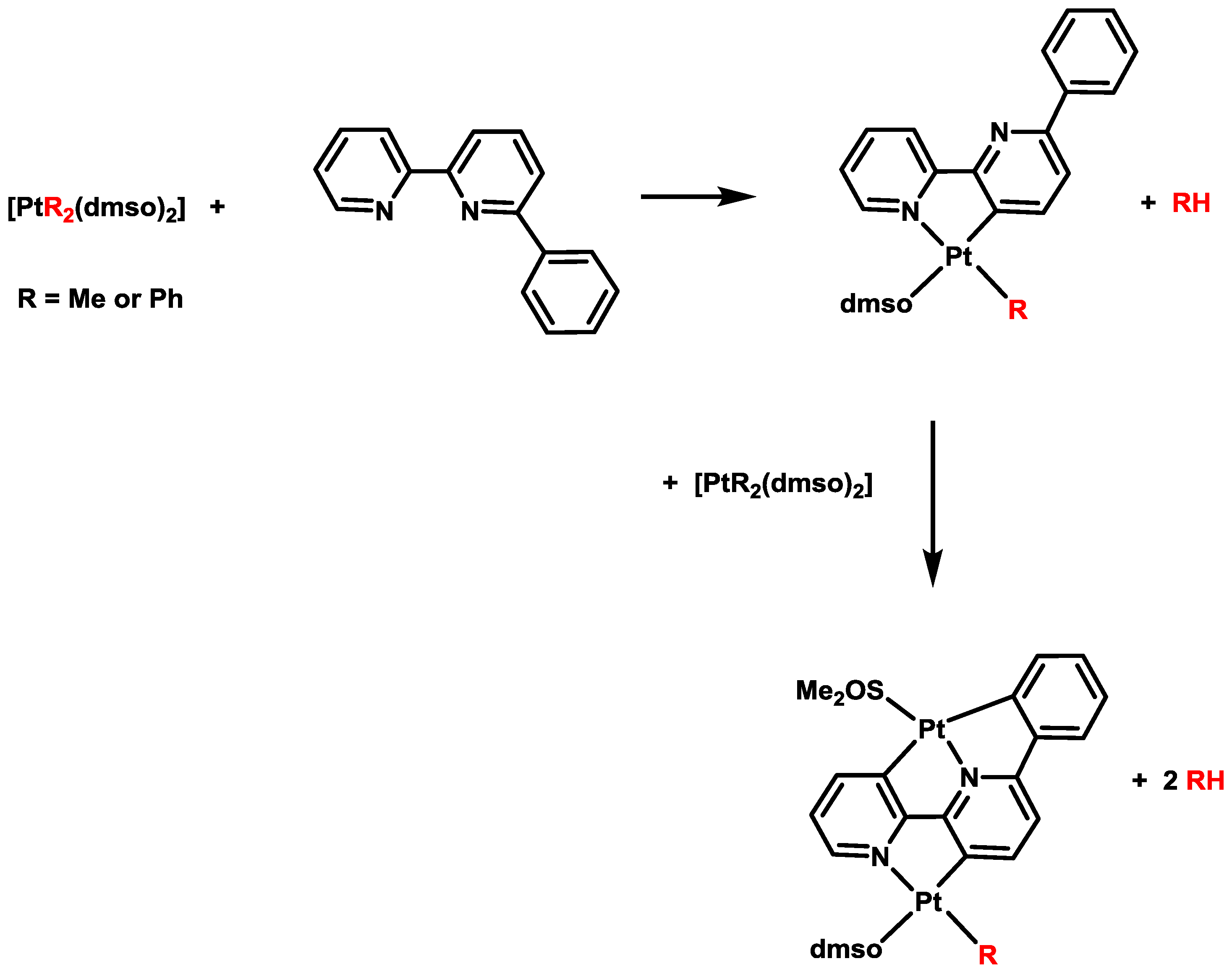
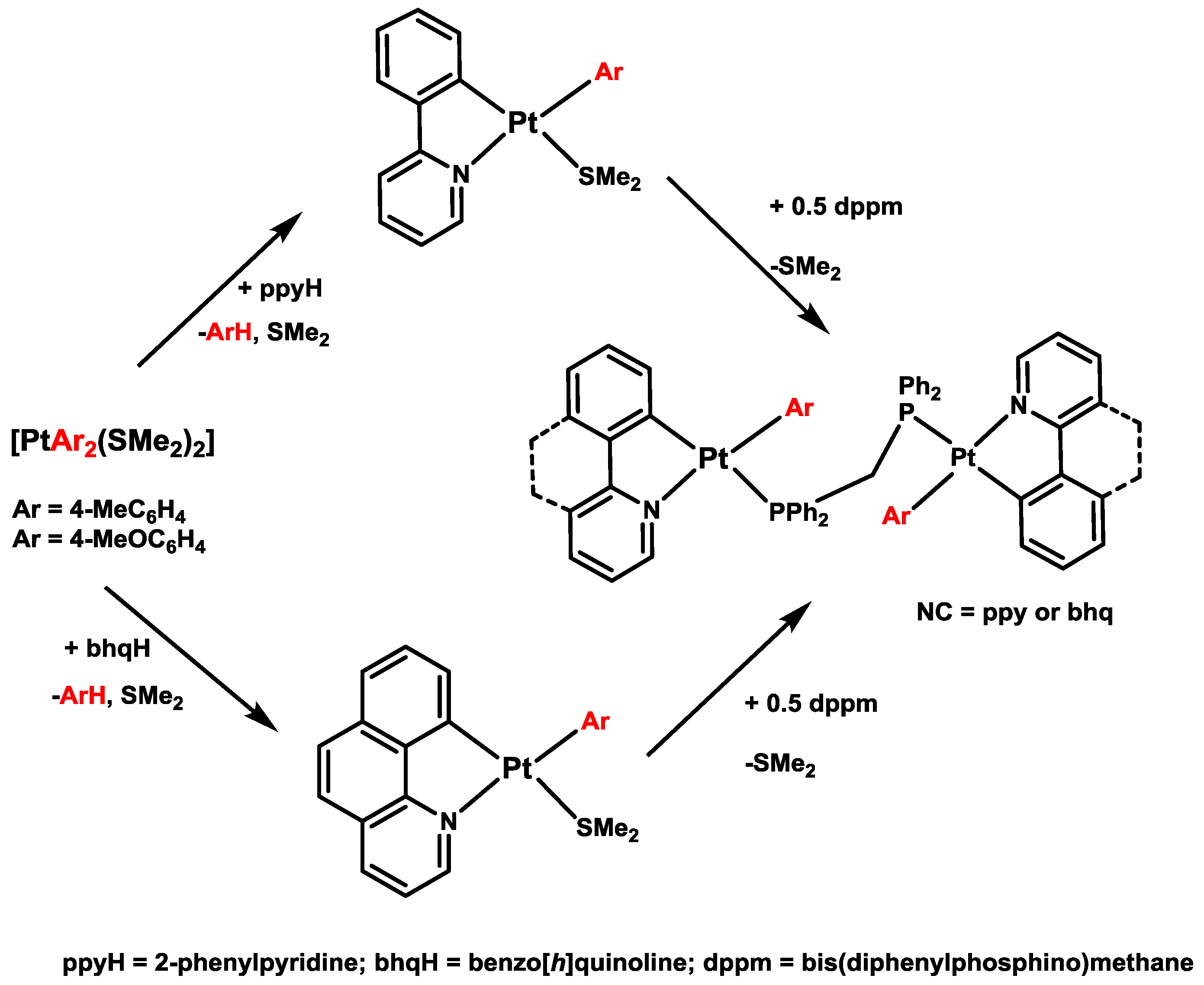
2. Activation of C–H Bonds at Diarylplatinum(II) Precursors
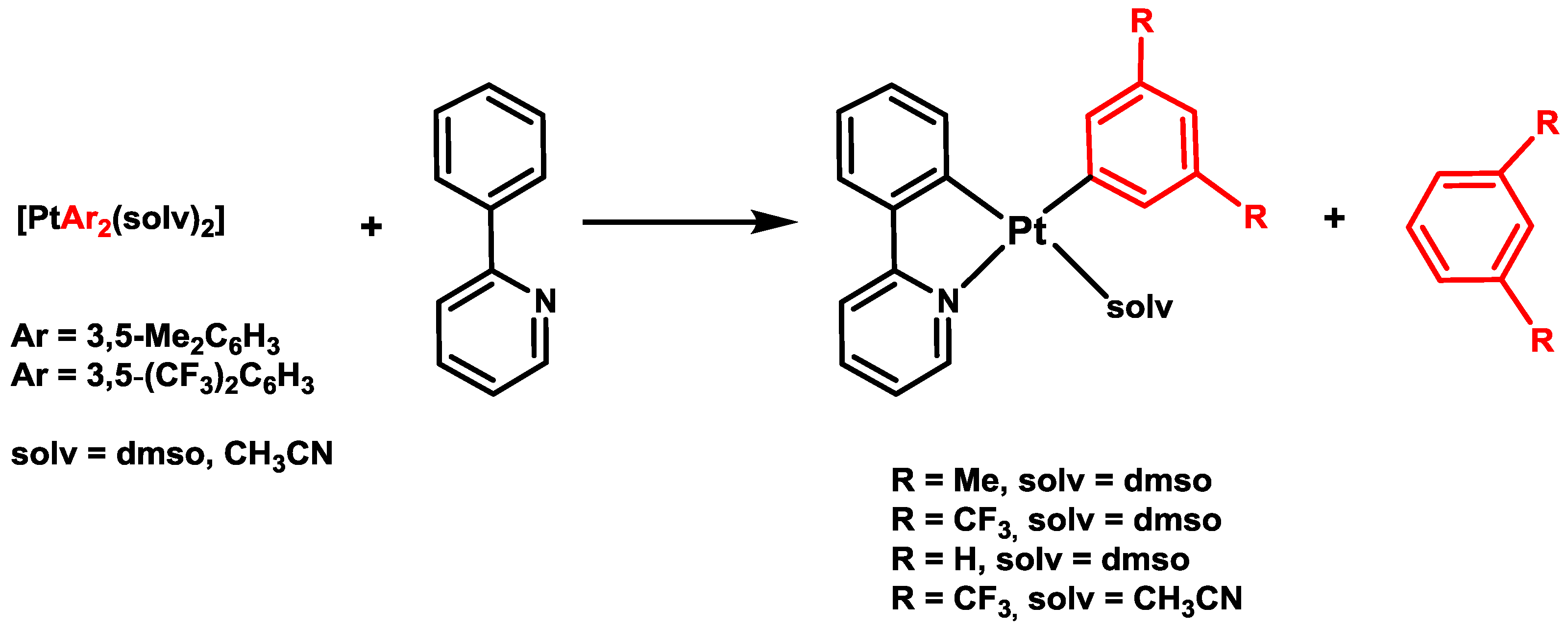
3. Activation of C–X Bonds (X = Br, Cl or H) at Diarylplatinum(II) Precursors: Seven- versus Five-Membered Platinacycles
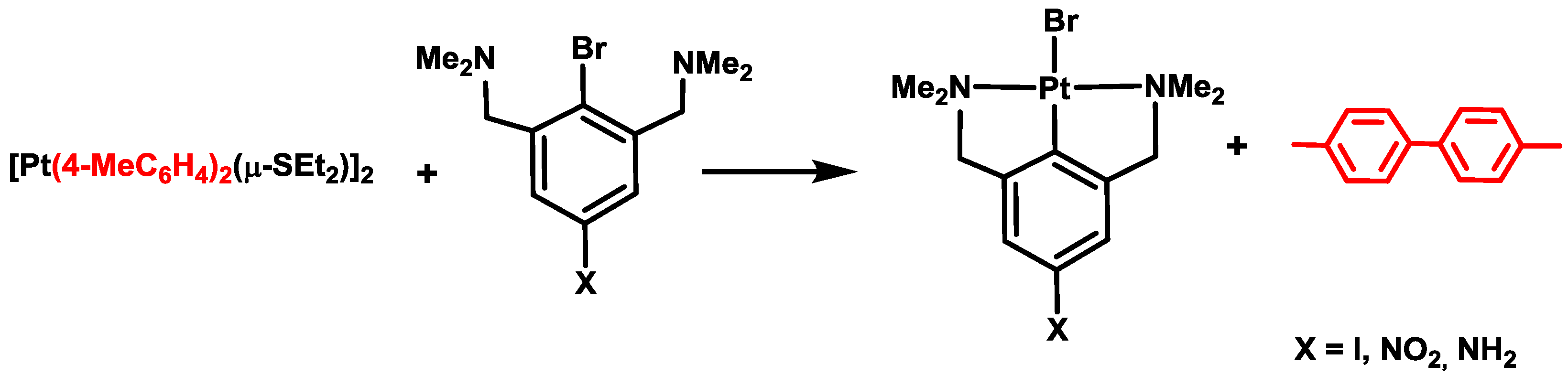
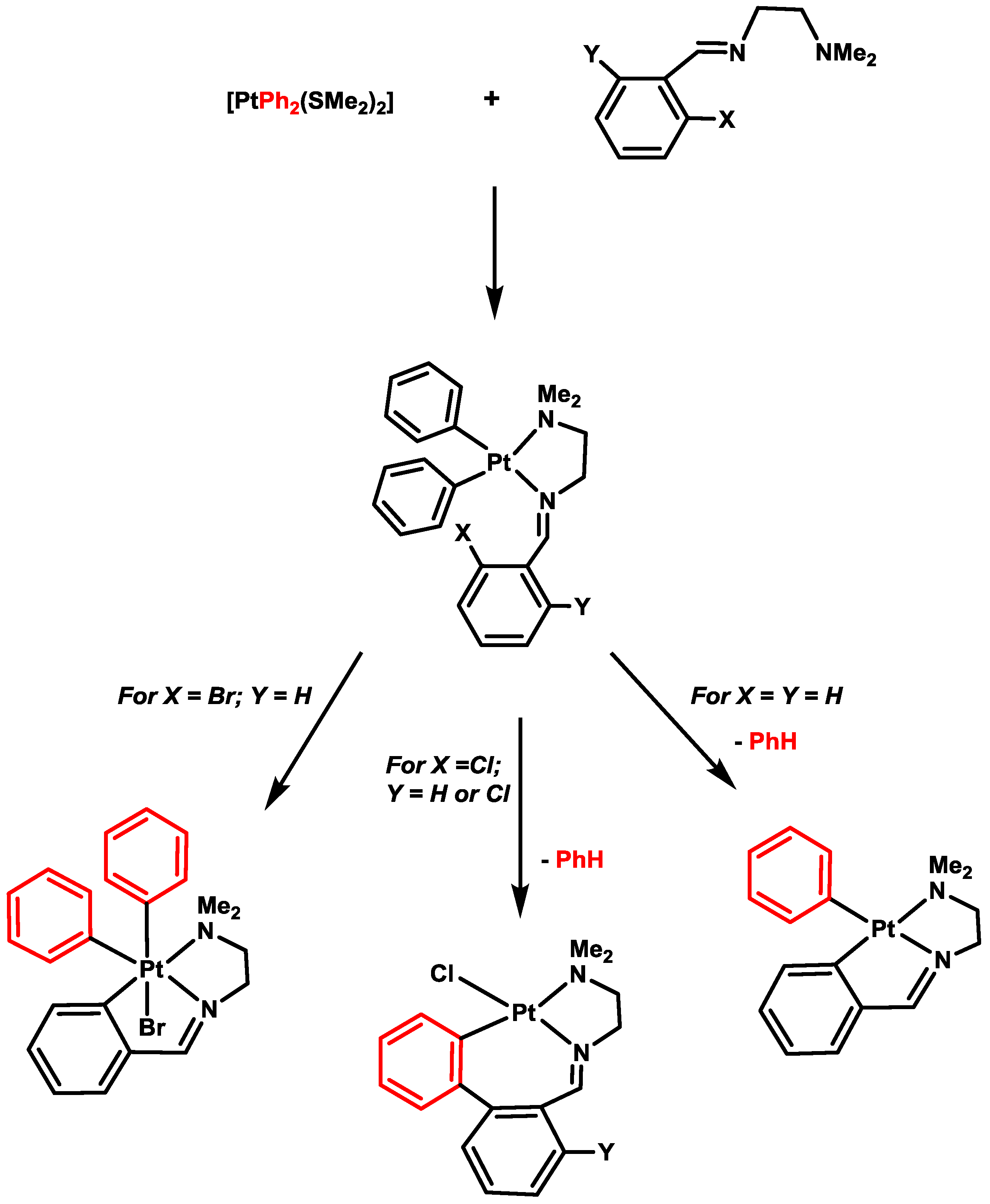


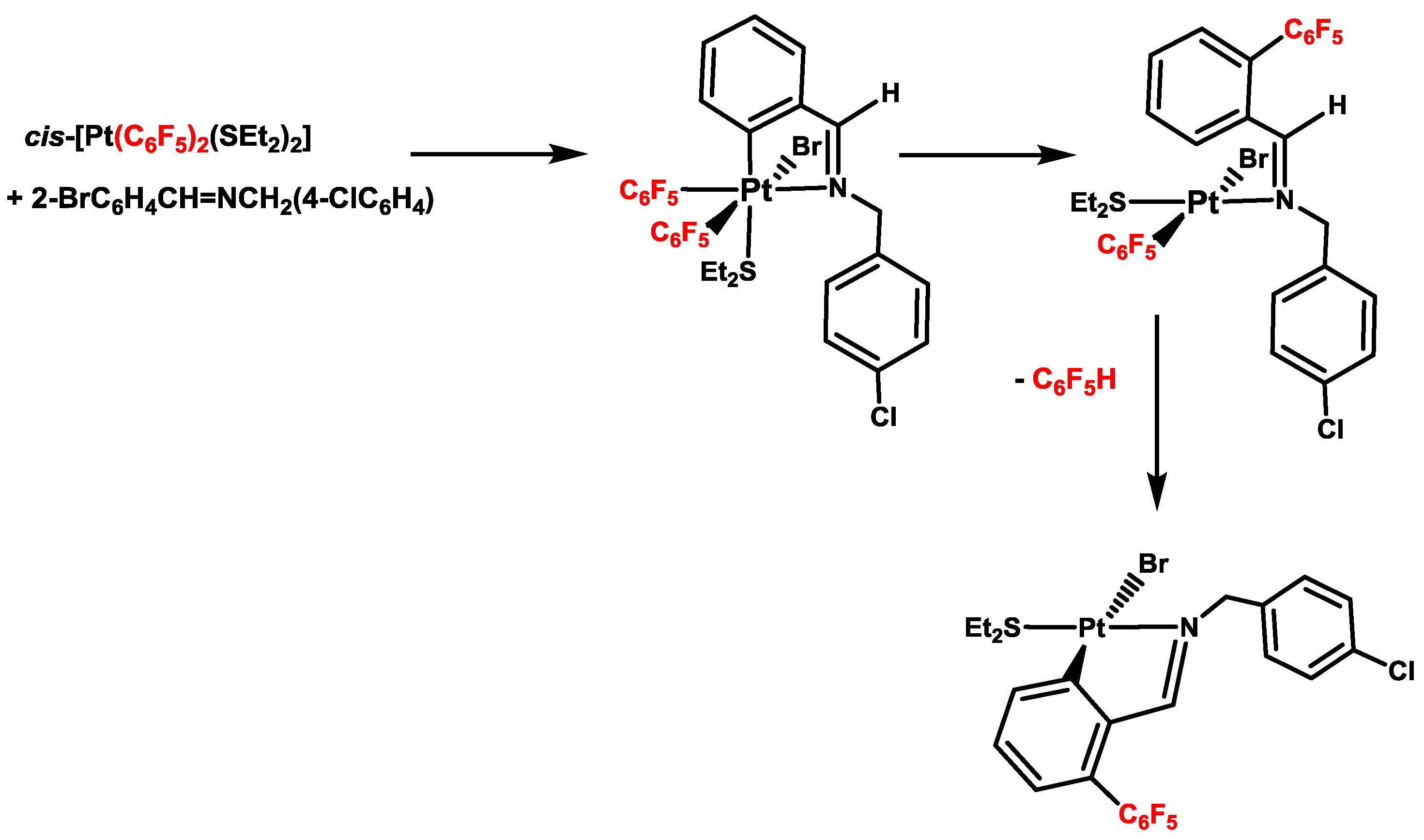
4. Six- versus Five-Membered and endo- versus exo-Platinacycles

5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Williams, J.A.G.; Develay, S.; Rochester, D.L.; Murphy, L. Optimising the luminescence of platinum(II) complexes and their application in organic light emitting devices (OLEDs). Coord. Chem. Rev. 2008, 252, 2596–2611. [Google Scholar] [CrossRef]
- Kalinowski, J.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Light-emitting devices based on organometallic platinum complexes as emitters. Coord. Chem. Rev. 2011, 255, 2401–2425. [Google Scholar] [CrossRef]
- Murphy, L.; Brulatti, P.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Blue-shifting of the monomer and excimer phosphorescence of tridentate cyclometallated platinum(II) complexes for optimal white-light OLEDs. Chem. Commun. 2012, 48, 5817–5819. [Google Scholar] [CrossRef]
- Kui, S.C.F.; Hung, F.-F.; Lai, S.-L.; Yuen, M.-Y.; Kwok, C.-C.; Low, K.-H.; Chui, S.S.-Y.; Che, C.-M. Luminescent organoplatinum(II) complexes with functionalized cyclometalated CNC ligands: Structures, photophysical properties, and material applications. Chem. Eur. J. 2012, 18, 96–109. [Google Scholar] [CrossRef]
- Zhang, L.-K.; Xing, L.-B.; Chen, B.; Yang, Q.-Z.; Tong, Q.-X.; Wu, L.-Z.; Tung, C.-H. A highly selective and sensitive luminescent chemosensor for Zn2+ ions based on cyclometalated platinum(II) complexes. Dalton Trans. 2013, 42, 4244–4247. [Google Scholar] [CrossRef]
- Li, Z.; Badaeva, E.; Ugrinov, A.; Kilina, S.; Sun, W. Platinum chloride complexes containing 6-[9,9-di(2-ethylhexyl)-7-R-9H-fluoren-2-yl]-2,2'-bipyridine ligand (R = NO2, CHO, benzothiazol-2-yl, n-Bu, carbazol-9-yl, NPh2): Tunable Photophysics and reverse saturable absorption. Inorg. Chem. 2013, 52, 7578–7592. [Google Scholar] [CrossRef]
- Turner, E.; Bakken, N.; Li, J. Cyclometalated platinum complexes with luminescent quantum yields approaching 100%. Inorg. Chem. 2013, 52, 7344–7351. [Google Scholar] [CrossRef]
- Li, Z.; Sun, W. Synthesis, photophysics, and reverse saturable absorption of platinum complexes bearing extended π-conjugated C^N^N ligands. Dalton Trans. 2013, 42, 14021–14029. [Google Scholar] [CrossRef]
- Samouei, H.; Rashidi, M.; Heinemann, F.W. A cyclometalated diplatinum complex containing 1,1'-bis(diphenylphosphino)ferrocene as spacer ligand: Antitumor study. J. Organomet. Chem. 2011, 696, 3764–3771. [Google Scholar] [CrossRef]
- Wang, P.; Leung, C.-H.; Ma, D.-L.; Sun, R.W.-Y.; Yan, S.-C.; Chen, Q.-S.; Che, C.-M. Specific blocking of CREB/DNA binding by cyclometalated platinum(II) complexes. Angew. Chem. Int. Ed. 2011, 50, 2554–2558. [Google Scholar] [CrossRef]
- Ruiz, J.; Vicente, C.; de Haro, C.; Espinosa, A. Synthesis and antiproliferative activity of a C,N-cycloplatinated(II) complex with a potentially intercalative anthraquinone pendant. Inorg. Chem. 2011, 50, 2151–2158. [Google Scholar] [CrossRef]
- Ruiz, J.; Rodríguez, V.; Cutillas, N.; Espinosa, A.; Hannon, M.J. Novel C,N-chelate platinum(II) antitumor complexes bearing a lipophilic ethisterone pendant. J. Inorg. Biochem. 2011, 105, 525–531. [Google Scholar] [CrossRef]
- Quirante, J.; Ruiz, D.; González, A.; López, C.; Cascante, M.; Cortés, R.; Messeguer, R.; Calvis, C.; Baldomà, L.; Pascual, A.; et al. Platinum(II) and palladium(II) complexes with (N,N′) and (C,N,N′)− ligands derived from pyrazole as anticancer and antimalarial agents: Synthesis, characterization and in vitro activities. J. Inorg. Biochem. 2011, 105, 1720–1728. [Google Scholar] [CrossRef]
- Chellan, P.; Land, K.M.; Shokar, A.; Au, A.; An, S.H.; Clavel, C.M.; Dyson, P.J.; de Kock, C.; Smith, P.J.; Chibale, K.; et al. Exploring the versatility of cycloplatinated thiosemicarbazones as antitumor and antiparasitic agents. Organometallics 2012, 31, 5791–5799. [Google Scholar] [CrossRef]
- Cortés, R.; Crespo, M.; Davin, L.; Martín, R.; Quirante, J.; Ruiz, D.; Messeguer, R.; Calvis, C.; Baldomà, L.; Badia, J.; et al. Seven-membered cycloplatinated complexes as a new family of anticancer agents. X-ray characterization and preliminary biological studies. Eur. J. Med. Chem. 2012, 54, 557–566. [Google Scholar] [CrossRef]
- Talancón, D.; López, C.; Font-Bardia, M.; Calvet, T.; Quirante, J.; Calvis, C.; Messeguer, R.; Cortés, R.; Cascante, M.; Baldomà, L.; et al. Diastereomerically pure platinum(II) complexes as antitumoral agents. The influence of the mode of binding {(N), (N,O)− or (C,N)−} of (1S,2R)-[(η5-C5H5)Fe{(η5-C5H4)–CH=N–CH(Me)–CH(OH)–C6H5}] and the arrangement of the auxiliary ligands. J. Inorg. Biochem. 2013, 118, 1–12. [Google Scholar] [CrossRef]
- Albert, J.; Bosque, R.; Crespo, M.; Granell, J.; López, C.; Cortés, R.; González, A.; Quirante, J.; Calvis, C.; Messeguer, R.; et al. Pt(II) complexes with (N,N') or (C,N,E)− (E = N, S) ligands: Cytotoxic studies, effect on DNA tertiary structure and structure-activity relationships. Bioorg. Med. Chem. 2013, 21, 4210–4217. [Google Scholar] [CrossRef]
- Albrecht, M. Cyclometalation using d-block transition metals: Fundamental aspects and recent trends. Chem. Rev. 2010, 110, 576–623. [Google Scholar] [CrossRef]
- Albrecht, M. C–H bond activation. In Palladacycles; Dupont, J., Pfeffer, M., Eds.; Wiley-VCH: Weinheim, Germany, 2008; Chapter 2; pp. 13–31. [Google Scholar]
- Ranatunge-Bandarage, P.R.R.; Robinson, B.H.; Simpson, J. Ferrocenylamine complexes of platinum(II) including cycloplatinated derivatives. Organometallics 1994, 13, 500–510. [Google Scholar] [CrossRef]
- Ranatunge-Bandarage, P.R.R.; Duffy, N.W.; Johnston, S.M.; Robinson, B.H.; Simpson, J. Synthesis and stereochemistry of bis(platinum) complexes of ferrocenylamines. Organometallics 1994, 13, 511–521. [Google Scholar] [CrossRef]
- Wu, Y.J.; Ding, L.; Wang, H.X.; Liu, Y.H.; Yuan, H.Z.; Mao, X.A. Synthesis, characterization and structure of ferrocenylketimine complexes of platinum(II). J. Organomet. Chem. 1997, 535, 49–58. [Google Scholar] [CrossRef]
- Ryabov, A.D.; Kazankov, G.M.; Panyashkina, I.M.; Grozovsky, O.V.; Dyachenko, O.G.; Polyakov, V.A.; Kuz’mina, L.G. Cycloplatination of aryl and ferrocenyl oximes by cis-[PtCl2(SOMe2)2] affording expected platinum(II) and unexpected platinum(IV) compounds. J. Dalton Trans. 1997, 4385–4391. [Google Scholar]
- Ding, L.; Zou, D.P.; Wu, Y.J. Cyclometallation reaction of 1,1'-bis[1-aryliminoethyl]ferrocenes with platinum(II). Polyhedron 1998, 17, 2511–2516. [Google Scholar] [CrossRef]
- Alexandrova, L.; D’yachenko, O.G.; Kazankov, G.M.; Polyakov, V.A.; Samuleev, P.V.; Sansores, E.; Ryabov, A.D. Mechanism of biologically relevant deoxygenation of dimethylsulfoxide coupled with Pt(II) to Pt(IV) oxidation of orthoplatinated oximes. Synthetic, kinetic, electrochemical, X-ray structural and density functional study. J. Am. Chem. Soc. 2000, 122, 5189–5200. [Google Scholar] [CrossRef]
- Meijer, M.D.; Kleij, A.W.; Lutz, M.; Spek, A.L.; van Koten, G. Synthesis and characterization of platinum(II)-terminated dendritic carbosilanes: X-ray crystal structure of the model species [PtCl(C6H3{CH2NMe2}-2-SiMe3–5)(PPh3)]. J. Organomet. Chem. 2001, 621, 190–196. [Google Scholar] [CrossRef]
- Ryabov, A.D.; Panyashkina, I.M.; Polyakov, V.A.; Fisher, A. Access to central carbon chirality through cycloplatination of 1-(2-pyridinylthio)propanone by cis-[PtCl2(S-SOMe(p-tolyl))]. Organometallics 2002, 21, 1633–1636. [Google Scholar] [CrossRef]
- Ryabov, A.D.; Otto, S.; Samuleev, P.V.; Polyakov, V.A.; Alexandrova, L.; Kazankov, G.M.; Shova, S.; Revenco, M.; Lipkowski, J.; Johansson, M. Structural and mechanistic look at the orthoplatination of aryl oximes by dichlorobis(sulfoxide or sulfide)platinum(II) complexes. Inorg. Chem. 2002, 41, 4286–4294. [Google Scholar] [CrossRef]
- Crespo, M.; Font-Bardia, M.; Granell, J.; Martínez, M.; Solans, X. Cyclometallation on platinum(II) complexes; the role of the solvent and added base donor capability on the reaction mechanisms. Dalton Trans. 2003, 3763–3769. [Google Scholar]
- Capapé, A.; Crespo, M.; Granell, J.; Font-Bardia, M.; Solans, X. A comparative study of the structures and reactivity of cyclometallated platinum compounds of N-benzylidenebenzylamines and cycloplatination of a primary amine. Dalton Trans. 2007, 2030–2039. [Google Scholar]
- Zucca, A.; Doppiu, A.; Cinellu, M.A.; Stoccoro, S.; Minghetti, G.; Manassero, M. Multiple C–H bond activation. Threefold-deprotonated 6-Phenyl-2,2'-bipyridine as a bridging ligand in dinuclear platinum(II) derivatives. Organometallics 2002, 21, 783–785. [Google Scholar] [CrossRef]
- Minghetti, G.; Stoccoro, S.; Cinellu, M.A.; Soro, B.; Zucca, A. Activation of a C-H bond in a pyridine ring. Reaction of 6-Substituted 2,2'-Bipyridines with methyl and phenyl platinum(II) derivatives: N',C(3)-“rollover” cyclometalation. Organometallics 2003, 22, 4770–4777. [Google Scholar] [CrossRef]
- Zucca, A.; Petretto, G.L.; Stoccoro, S.; Cinellu, M.A.; Minghetti, G.; Manassero, M.; Manassero, C.; Male, L.; Albinati, A. Dinuclear C,N,C cyclometalated platinum derivatives with bridging delocalized ligands. fourfold deprotonation of 6,6'-Diphenyl-2,2'-bipyridine, H4L, promoted by “Pt(R)2” fragments (R = Me, Ph). Crystal Structures of [Pt2(L)(3,5-Me2py)2] and {Pt2(L)(dppe)}2 (dppe = 1,2-bis(diphenylphosphino)ethane). X-ray powder diffraction of [Pt2(L)(CO)2]. Organometallics 2006, 25, 2253–2265. [Google Scholar] [CrossRef]
- Zucca, A.; Petretto, G.L.; Stoccoro, S.; Cinellu, M.A.; Manassero, M.; Manassero, C.; Minghetti, G. Cyclometalation of 2,2'-bipyridine. Mono- and dinuclear C,N platinum(II) derivatives. Organometallics 2009, 28, 2150–2159. [Google Scholar] [CrossRef]
- Zucca, A.; Cordeschi, D.; Stoccoro, S.; Cinellu, M.A.; Minghetti, G.; Chelucci, G.; Manassero, M. Platinum(II)-cyclometalated “roll-over” complexes with a chiral pinene-derived 2,2'-bipyridine. Organometallics 2011, 30, 3064–3074. [Google Scholar] [CrossRef]
- Maidich, L.; Zucca, A.; Clarkson, G.J.; Rourke, J.P. Oxidative addition to diplatinum(II) complexes: Stereoselectivity and cooperative effects. Organometallics 2013, 32, 3371–3375. [Google Scholar] [CrossRef]
- Baar, C.R.; Jenkins, H.A.; Vital, J.J.; Yap, G.P.A.; Puddephatt, R.J. Stereoselectivity in organometallic reactions: Oxidative addition of alkyl halides to platinum(II). Organometallics 1998, 17, 2805–2818. [Google Scholar] [CrossRef]
- Baar, C.R.; Carbray, L.P.; Jennings, M.C.; Puddephatt, R.J. Oxidative addition to diplatinum(II) complexes: Stereoselectivity and cooperative effects. Organometallics 2000, 19, 2482–2497. [Google Scholar] [CrossRef]
- Zhao, S.-B.; Wang, R.-Y.; Wang, S. Intramolecular C–H activation directed self-assembly of an organoplatinum(II) molecular square. J. Am. Chem. Soc. 2007, 129, 3092–3093. [Google Scholar] [CrossRef]
- Zhao, S.-B.; Wang, R.-Y.; Wang, S. Reactivity of SiMe3- and SnR3-functionalized bis(7-azaindol-1-yl)methane with [PtR2(μ-SMe2)]n (R = Me, Ph) and the resulting Pt(II) and Pt(IV) complexes. Organometallics 2009, 28, 2572–2582. [Google Scholar] [CrossRef]
- Anderson, C.M.; Puddephatt, R.J.; Ferguson, G.; Lough, A.J. Oxidative addition of aryl-halogen bonds to platinum(II) and the structure of a complex formed by aryl-fluoride oxidative addition. J. Chem. Commun. 1989, 1297–1298. [Google Scholar]
- Anderson, C.M.; Crespo, M.; Ferguson, G.; Lough, A.J.; Puddephatt, R.J. Activation of aromatic carbon-fluorine bonds by organoplatinum complexes. Organometallics 1992, 11, 1177–1181. [Google Scholar] [CrossRef]
- Anderson, C.M.; Crespo, M.; Jennings, M.C.; Lough, A.J.; Ferguson, G.; Puddephatt, R.J. Competition between intramolecular oxidative addition and ortho metalation in organoplatinum(II) compounds: Activation of aryl-halogen bonds. Organometallics 1991, 10, 2672–2679. [Google Scholar] [CrossRef]
- Rao, Y.-L.; Wang, S. Impact of constitutional isomers of (BMes2)phenylpyridine on structure, stability, phosphorescence, and Lewis acidity of mononuclear and dinuclear Pt(II) complexes. Inorg. Chem. 2009, 48, 7698–7713. [Google Scholar] [CrossRef]
- Yagyu, T.; Ohashi, J.; Maeda, M. Monoarylplatinum(II) complexes with a 2-phenylpyridyl ligand and coordinated solvent, [Pt(Ar)(Phpy)(solv)] (Phpy = 2-phenylpyridyl; solv = NCCH3, dmso). Preparation from [Pt(Ar)2(solv)2], structures, and chemical properties. Organometallics 2007, 26, 2383–2391. [Google Scholar] [CrossRef]
- Nabavizadeh, S.M.; Amini, H.; Shahsavari, H.R.; Namdar, M.; Rashidi, M.; Kia, R.; Hemmateenejad, B.; Nekoeinia, M.; Ariafard, A.; Hosseini, F.N.; et al. Assembly of cyclometalated platinum(II) complexes via 1,10-bis(diphenylphosphino)ferrocene ligand: Kinetics and mechanisms. Organometallics 2011, 30, 1466–1477. [Google Scholar] [CrossRef]
- Crespo, M.; Font-Bardia, M.; Solans, X. A comparative study of metallating agents in the synthesis of [C,N,N']-cycloplatinated compounds derived from biphenylimines. J. Organomet. Chem. 2006, 691, 1897–1906. [Google Scholar] [CrossRef]
- Crespo, M.; Anderson, C.M.; Tanski, J.M. Synthesis of platinum(II) cyclometallated compounds derived from imines containing pyridyl or pyrimidyl groups. Can. J. Chem. 2009, 87, 80–87. [Google Scholar] [CrossRef]
- Hudson, Z.M.; Zhao, S.-B.; Wang, R.-Y.; Wang, S. Switchable ambient temperature singlet-triplet dual emission in nonconjugated donor-acceptor triarylboron-Pt(II) complexes. Chem. Eur. J. 2009, 15, 6131–6137. [Google Scholar] [CrossRef]
- Skapski, A.C.; Sutcliffe, V.F.; Young, G.B. ‘Roll-over’ 3-metallation of co-ordinated 2,2'-bipyridyl in the thermal rearrangement of diaryl(bipyridyl)platinum(II) complexes: Molecular structure of (μ-bidyl)[PtPh(Butpy)]2. J. Chem. Soc. Chem. Commun. 1985, 609–611. [Google Scholar] [CrossRef]
- Nabavizadeh, S.M.; Haghighi, M.G.; Esmaeilbeig, A.R.; Raoof, F.; Mandegani, Z.; Jamali, S.; Rashidi, M.; Puddephatt, R.J. Assembly of symmetrical or unsymmetrical cyclometalated organoplatinum complexes through a bridging diphosphine ligand. Organometallics 2010, 29, 4893–4899. [Google Scholar] [CrossRef]
- Nabavizadeh, S.M.; Shahsavari, H.; Namdar, M.; Rashidi, M.; Puddephatt, R.J. Substitution reactions involving cyclometalated platinum(II) complexes: Kinetic investigations. J. Organomet. Chem. 2011, 696, 3564–3571. [Google Scholar] [CrossRef]
- Forniés, J.; Menjón, B.; Gómez, N.; Tomás, M. Reactivity of cis-Pt(C6F5)2(OC4H8)2 toward (C6H5)2CN2. Synthesis and molecular structure of Pt(C6F5)[(2-C6H4)C(C6H5)=N–N=(η2-C6H5)C(C6H5)], an ortho-metalated compound containing an unusual intramolecular η2-Arene-Pt interaction. Organometallics 1992, 11, 1187–1193. [Google Scholar] [CrossRef]
- Berenguer, J.R.; Lalinde, E.; Moreno, M.T.; Sánchez, S.; Torroba, J. Facile metalation of Hbzq by cis-[Pt(C6F5)2(thf)2]: A route to a pentafluorophenyl benzoquinolate solvate complex that easily coordinates terminal alkynes. Spectroscopic and optical properties. Inorg. Chem. 2012, 51, 11665–11669. [Google Scholar] [CrossRef]
- Sarkar, B.; Schurr, T.; Hartenbach, I.; Shleid, T.; Fiedler, J.; Kaim, W. Double cyclometallation of bridging 3,6-bis(2-thienyl)-1,2,4,5-tetrazine in a dinuclear mesityl(dimethylsulfoxide)platinum(II) complex: Structure and properties. J. Organomet. Chem. 2008, 693, 1703–1706. [Google Scholar] [CrossRef]
- Rodríguez, G.; Albrecht, M.; Schoenmaker, J.; Ford, A.; Lutz, M.; Spek, A.L.; van Koten, G. Bifunctional pincer-type organometallics as substrates for organic transformations and as novel building blocks for polymetallic materials. J. Am. Chem. Soc. 2002, 124, 5127–5138. [Google Scholar] [CrossRef]
- Albrecht, M.; Rodríguez, G.; Schoenmaker, J.; van Koten, G. New peptide labels containing covalently bonded platinum(II) centers as diagnostic biomarkers and biosensors. Org. Lett. 2000, 2, 3461–3464. [Google Scholar] [CrossRef]
- Canty, A.J.; Patel, J.; Skelton, B.W.; White, A.H. Facial and meridional [N–C–N]− intramolecular coordination systems: Structure of fac-PtBrMe2{2,6-(pzCH2)2C6H3}·1/2C6H6 {[2,6-(pzCH2)2C6H3]− = 2,6-(bis{(pyrazol-1-yl)methyl}phenyl)} and mer-PtBr{2,6-(3,5-Me2pzCH2)2C6H3}, and an alternative synthetic route to the platinum(II) [N–C–N]− kernel. J. Organomet. Chem. 2000, 599, 195–199. [Google Scholar] [CrossRef]
- Slagt, M.Q.; Gebbink, R.J.M.K.; Lutz, M.; Spek, A.L.; van Koten, G. Synthetic strategies towards new para-functionalised NCN-pincer palladium(II) and platinum(II) complexes. Dalton Trans. 2002, 2591–2592. [Google Scholar]
- Crespo, M.; Font-Bardia, M.; Solans, X. Compound [PtPh2(SMe2)2] as a versatile metallating agent in the preparation of new types of [C,N,N'] cyclometallated platinum compounds. Organometallics 2004, 23, 1708–1713. [Google Scholar] [CrossRef]
- Calvet, T.; Crespo, M.; Font-Bardia, M.; Jansat, S.; Martinez, M. Kinetico-mechanistic studies on intramolecular C–X bond activation (X = Br, Cl) of amino-imino ligands on Pt(II) compounds. Prevalence of a concerted mechanism in nonpolar, polar and ionic liquid media. Organometallics 2012, 31, 4367–4373. [Google Scholar] [CrossRef]
- Martín, R.; Crespo, M.; Font-Bardia, M.; Calvet, T. Five- and seven-membered metallacycles in [C,N,N'] and [C,N] cycloplatinated compounds. Organometallics 2009, 28, 587–597. [Google Scholar] [CrossRef]
- Bernhardt, P.V.; Calvet, T.; Crespo, M.; Font-Bardia, M.; Jansat, S.; Martínez, M. New insights in the formation of five- versus seven- membered platinacycles: A kinetico-mechanistic study. Inorg. Chem. 2013, 52, 474–484. [Google Scholar] [CrossRef]
- Font-Bardia, M.; Gallego, C.; Martinez, M.; Solans, X. Unexpected formal aryl insertion in a cyclometalated diphenylplatinum(IV) Complex: The first seven-membered cyclometalated platinum compound structurally characterized. Organometallics 2002, 21, 3305–3307. [Google Scholar] [CrossRef]
- Gallego, C.; Martinez, M.; Safont, V.S. Mechanism of the competition between phenyl insertion and ligand reductive elimination on a hindered platinum(IV) cyclometalated complex. Organometallics 2007, 26, 527–537. [Google Scholar] [CrossRef]
- Calvet, T.; Crespo, M.; Font-Bardia, M.; Gómez, K.; González, G.; Martínez, M. Kinetico-mechanistic insight into the platinum-mediated C–C coupling of fluorinated arenes. Organometallics 2009, 28, 5096–5106. [Google Scholar] [CrossRef]
- Crespo, M.; Calvet, T.; Font-Bardia, M. Platinum mediated aryl-aryl bond formation and sp3 C–H bond activation. Dalton Trans. 2010, 39, 6936–6938. [Google Scholar] [CrossRef]
- Crespo, M.; Font-Bardia, M.; Calvet, T. Biaryl formation in the synthesis of endo- and exo-platinacycles. Dalton Trans. 2011, 40, 9431–9438. [Google Scholar] [CrossRef]
- Albert, J.; Granell, J.; Sales, J.; Solans, X.; Font-Altaba, M. Competitive metalation reactions between aliphatic and aromatic carbon atoms in N-benzylideneamines. X-ray molecular structure of [Pd{1-CH2-2-(HC=NC6H5)-3,5-(CH3)2C6H2)BrPPh3]. Organometallics 1986, 5, 2567–2568. [Google Scholar] [CrossRef]
- Albert, J.; Ceder, R.M.; Gomez, M.; Granell, J.; Sales, J. Cyclopalladation of N-mesitylbenzylideneamines. Aromatic versus aliphatic C–H activation. Organometallics 1992, 11, 1536–1541. [Google Scholar] [CrossRef]
- Crespo, M.; Anderson, C.M.; Kfoury, N.; Font-Bardia, M.; Calvet, T. Reductive elimination from cyclometalated platinum(IV) complexes to form Csp2–Csp3 bonds and subsequent competition between Csp2–H and Csp3–H bond activation. Organometallics 2012, 31, 4401–4404. [Google Scholar] [CrossRef]
- Anderson, C.M.; Crespo, M.; Kfoury, N.; Weinstein, M.A.; Tanski, J.M. Regioselective C–H activation preceded by Csp2–Csp3 reductive elimination from cyclometalated platinum(IV) complexes. Organometallics 2013, 32, 4199–4207. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Crespo, M. Diarylplatinum(II) Compounds as Versatile Metallating Agents in the Synthesis of Cyclometallated Platinum Compounds with N-Donor Ligands. Inorganics 2014, 2, 115-131. https://doi.org/10.3390/inorganics2010115
Crespo M. Diarylplatinum(II) Compounds as Versatile Metallating Agents in the Synthesis of Cyclometallated Platinum Compounds with N-Donor Ligands. Inorganics. 2014; 2(1):115-131. https://doi.org/10.3390/inorganics2010115
Chicago/Turabian StyleCrespo, Margarita. 2014. "Diarylplatinum(II) Compounds as Versatile Metallating Agents in the Synthesis of Cyclometallated Platinum Compounds with N-Donor Ligands" Inorganics 2, no. 1: 115-131. https://doi.org/10.3390/inorganics2010115