Is Cell Death Primary or Secondary in the Pathophysiology of Idiopathic Parkinson’s Disease?
Abstract
:1. Do Lewy Bodies Cause Cell Death?
2. Clinical Findings Suggest Synaptic Pathology of Still-Existing Neurons in Parkinson’s Disease
3. An Approach to Explain Neurodegeneration in α-Synuclein Aggregation Diseases other than by Lewy Bodies and Cell Death
4. Link between Synaptic α-Synuclein Aggregation and Synaptic Failure
5. Neuronal Dysfunction, Lewy Body Formation and Cell Death
6. Animal models in Parkinson’s disease
7. Pathophysiological Cascade Leading to Neurodegeneration in PD/DLB
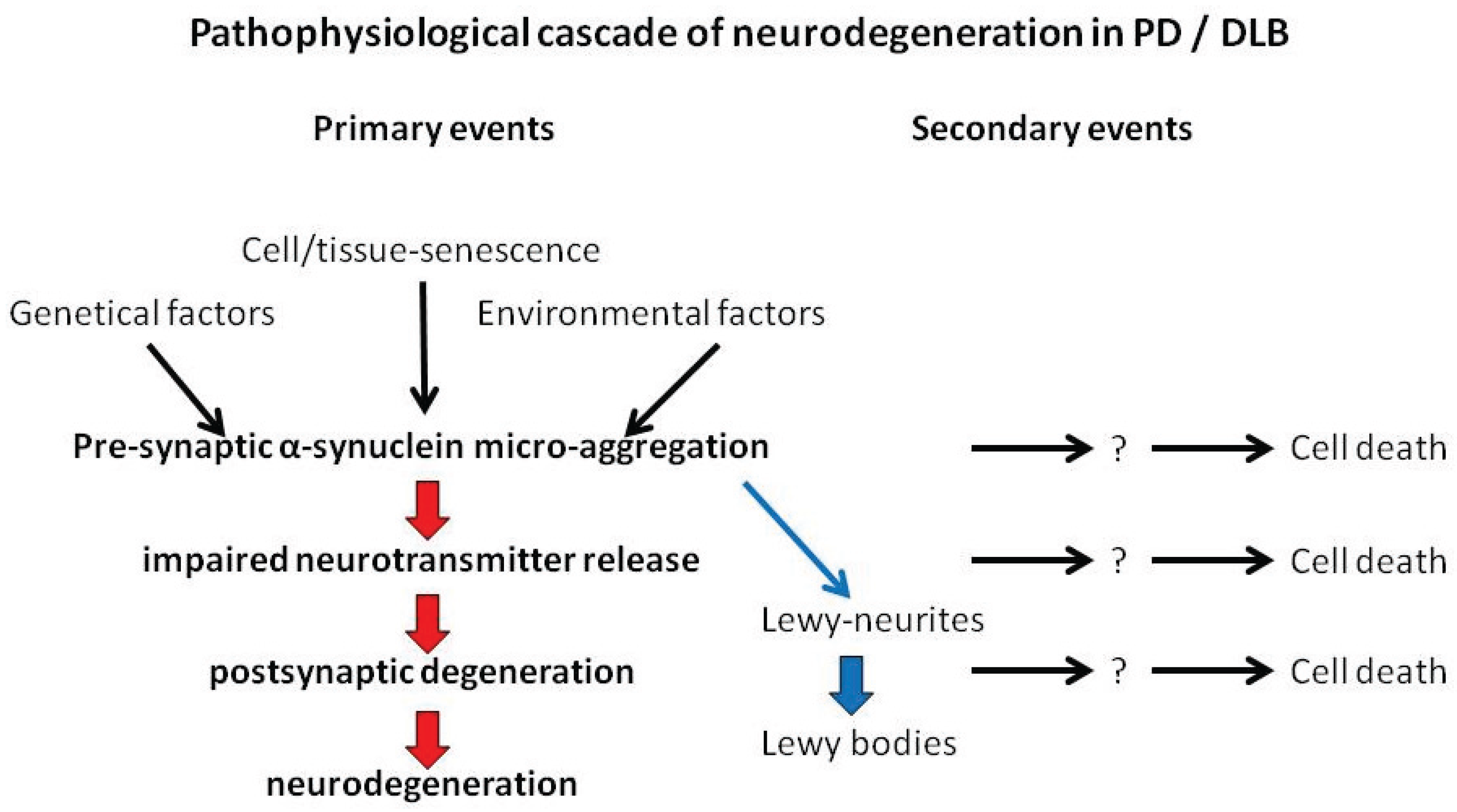
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- George, S.; Rey, N.L.; Reichenbach, N.; Steiner, J.A.; Brundin, P. α-Synuclein: The long distance runner. Brain Pathol. 2013, 23, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G. Is the Lewy body telling us anything useful about the pathogenesis of Parkinson’s disease?—To understand how the study of the Lewy body gives a profound insight into the pathogenesis of Parkinson’s disease. In Proceedings of the 17th International Congress of Parkinson’s Disease and Movement Disorders, Sydney, Australia, 17 June 2013.
- Dickson, D.W.; Fujishiro, H.; DelleDonne, A.; Menke, J.; Ahmed, Z.; Klos, K.J.; Josephs, K.A.; Frigerio, R.; Burnett, M.; Parisi, J.E.; et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol. 2008, 115, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Lewy body-related alpha-synucleinopathy in the aged human brain. J. Neural Transm. 2004, 111, 1219–1235. [Google Scholar] [CrossRef] [PubMed]
- Gibb, W.R.; Lees, A.J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008, 116, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Tanji, K.; Mori, F.; Takahashi, H. The Lewy body in Parkinson’s disease: Molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology 2007, 27, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Milber, J.M.; Noorigian, J.V.; Morley, J.F.; Petrovitch, H.; White, L.; Ross, G.W.; Duda, J.E. Lewy pathology is not the first sign of degeneration in vulnerable neurons in PD. Neurology 2012, 79, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, M.M.; Hill, W.D. Contribution of somal Lewy bodies to neuronal death. Brain Res. 1997, 775, 24–29. [Google Scholar] [CrossRef]
- Bergeron, C.; Petrunka, C.; Weyer, L.; Pollanen, M.S. Altered neurofilament expression does not contribute to Lewy body formation. Am. J. Pathol. 1996, 148, 267–272. [Google Scholar] [PubMed]
- Hill, W.D. Altered neurofilament expression does not contribute to Lewy body formation. Am. J. Pathol. 1996, 149, 728–729. [Google Scholar] [PubMed]
- Javoy-Agid, F.; Hirsch, E.C.; Dumas, S.; Duyckaerts, C.; Mallet, J.; Agid, Y. Decreased tyrosine hydroxylase messenger RNA in the surviving dopamine neurons of the substantia nigra in Parkinson’s disease: An in situ hybridization study. Neuroscience 1990, 38, 245–253. [Google Scholar] [CrossRef]
- Patt, S.; Gertz, H.J.; Gerhard, L.; Cervos-Navarro, J. Pathological changes in dendrites of substantia nigra neurons in Parkinson’s disease: A Golgi study. Histol. Histopathol. 1991, 6, 373–380. [Google Scholar] [PubMed]
- Gomez-Isla, T.; Growdon, W.B.; McNamara, M.; Newell, K.; Gomez-Tortosa, E.; Hedley-Whyte, E.T.; Hyman, B.T. Clinicopathologic correlates in temporal cortex in dementia with Lewy bodies. Neurology 1999, 53, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Tortosa, E.; Newell, K.; Irizarry, M.C.; Albert, M.; Growdon, J.H.; Hyman, B.T. Clinical and quantitative pathologic correlates of dementia with Lewy bodies. Neurology 1999, 53, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Tortosa, E.; Irizarry, M.C.; Gomez-Isla, T.; Hyman, B.T. Clinical and neuropathological correlates of dementia with Lewy bodies. Ann. NY Acad. Sci. 2000, 920, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.M.; Rinne, J.O.; Helenius, H.; Dickson, D.W.; Roytta, M. α-Synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol. 2000, 100, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Weisman, D.; Cho, M.; Taylor, C.; Adame, A.; Thal, L.J.; Hansen, L.A. In dementia with Lewy bodies, Braak stage determines phenotype, not Lewy body distribution. Neurology 2007, 69, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.J.; Halliday, G.M. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001, 102, 355–363. [Google Scholar] [PubMed]
- Harding, A.J.; Broe, G.A.; Halliday, G.M. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain 2002, 125, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.J.; Stimson, E.; Henderson, J.M.; Halliday, G.M. Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain 2002, 125, 2431–2445. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E.; Pollack, J.; Morris, J.C. Clinical phenotype of Parkinson disease dementia. Neurology 2006, 67, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Libow, L.S.; Frisina, P.G.; Haroutunian, V.; Perl, D.P.; Purohit, D.P. Parkinson’s disease dementia: A diminished role for the Lewy body. Parkinsonism Relat. Disord. 2009, 15, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Schaeffer, W.J. Neurodegeneration in Parkinson disease: Moving Lewy bodies out of focus. Neurology 2012, 79, 2298–2299. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Perl, D.P.; DeMartino, G.N.; McNaught, K.S. Lewy-body formation is an aggresome-related process: A hypothesis. Lancet Neurol. 2004, 3, 496–503. [Google Scholar] [CrossRef]
- Burre, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Cabin, D.E.; Shimazu, K.; Murphy, D.; Cole, N.B.; Gottschalk, W.; McIlwain, K.L.; Orrison, B.; Chen, A.; Ellis, C.E.; Paylor, R.; et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J. Neurosci. 2002, 22, 8797–8807. [Google Scholar] [PubMed]
- Chandra, S.; Fornai, F.; Kwon, H.B.; Yazdani, U.; Atasoy, D.; Liu, X.; Hammer, R.E.; Battaglia, G.; German, D.C.; Castillo, P.E.; et al. Double-knockout mice for α- and β-synucleins: Effect on synaptic functions. Proc. Natl. Acad. Sci. USA 2004, 101, 14966–14971. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.D.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M. Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 2000, 20, 3214–3220. [Google Scholar] [PubMed]
- Scott, D.; Roy, S. α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J. Neurosci. 2012, 32, 10129–10135. [Google Scholar] [CrossRef] [PubMed]
- DiRosa, G.; Puzzo, D.; Sant’Angelo, A.; Trinchese, F.; Arancio, O. α-Synuclein: Between synaptic function and dysfunction. Histol. Histopathol. 2003, 18, 1257–1266. [Google Scholar]
- Liu, S.; Ninan, I.; Antonova, I.; Battaglia, F.; Trinchese, F.; Narasanna, A.; Kolodilov, N.; Dauer, W.; Hawkins, R.D.; Arancio, O. alpha-Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J. 2004, 23, 4506–4516. [Google Scholar] [CrossRef] [PubMed]
- Al-Wandi, A.; Ninkina, N.; Millership, S.; Williamson, S.J.; Jones, P.A.; Buchman, V.L. Absence of alpha-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol. Aging 2010, 31, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Fortin, D.L.; Nemani, V.M.; Voglmaier, S.M.; Anthony, M.D.; Ryan, T.A.; Edwards, R.H. Neural activity controls the synaptic accumulation of alpha-synuclein. J. Neurosci. 2005, 25, 10913–10921. [Google Scholar] [CrossRef] [PubMed]
- Lotharius, J.; Brundin, P. Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson’s disease. Hum. Mol. Genet. 2002, 11, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.; Wersinger, C.; Vernier, P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J. 2004, 18, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Yavich, L.; Tanila, H.; Vepsalainen, S.; Jakala, P. Role of alpha-synuclein in presynaptic dopamine recruitment. J. Neurosci. 2004, 24, 11165–11170. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Hornykiewicz, O. Basic research on dopamine in Parkinson’s disease and the discovery of the nigrostriatal dopamine pathway: The view of an eyewitness. Neurodegener. Dis. 2008, 5, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Antke, C.; Müller, H.W. In vivo imaging of synaptic function in the central nervous system: I. Movement disorders and dementia. Behav. Brain Res. 2009, 204, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Linazasoro, G. Classical Parkinson disease versus Parkinson complex—Reflections against staging and in favour of heterogeneity. Eur. J. Neurol. 2007, 14, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Schaeffer, W.J.; Tschöke, S.; Kranefuss, N.; Dröse, W.; Hause-Reitner, D.; Giese, A.; Groschup, M.H.; Kretzschmar, H.A. The paraffin-embedded tissue blot detects PrP(Sc) early in the incubation time in prion diseases. Am. J. Pathol. 2000, 156, 51–56. [Google Scholar] [CrossRef]
- Kramer, M.L.; Schulz-Schaeffer, W.J. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J. Neurosci. 2007, 27, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Schaeffer, W.J. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010, 120, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Miake, H.; Mizusawa, H.; Iwatsubo, T.; Hasegawa, M. Biochemical characterization of the core structure of alpha-synuclein filaments. J. Biol. Chem. 2002, 277, 19213–19219. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.L.; Behrens, C.; Schulz-Schaeffer, W.J. Selective detection, quantification, and subcellular location of alpha-synuclein aggregates with a protein aggregate filtration assay. Biotechniques 2008, 44, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Haycock, J.W. Polyvinylpyrrolidone as a blocking agent in immunochemical studies. Anal. Biochem. 1993, 208, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Huttner, W.B.; Schiebler, W.; Greengard, P.; De, C.P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol. 1983, 96, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Revuelta, G.J.; Rosso, A.; Lippa, C.F. Neuritic pathology as a correlate of synaptic loss in dementia with lewy bodies. Am. J. Alzheimers Dis. Other Dement. 2008, 23, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.; Sekino, Y.; Hanamura, K.; Fujisawa, S.; Mahadomrongkul, V.; Ren, Y.; Shirao, T. Drebrin A is a postsynaptic protein that localizes in vivo to the submembranous surface of dendritic sites forming excitatory synapses. J. Comp. Neurol. 2005, 483, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Ingham, C.A.; Hood, S.H.; Arbuthnott, G.W. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res. 1989, 503, 334–338. [Google Scholar] [CrossRef]
- Solis, O.; Limon, D.I.; Flores-Hernandez, J.; Flores, G. Alterations in dendritic morphology of the prefrontal cortical and striatum neurons in the unilateral 6-OHDA-rat model of Parkinson’s disease. Synapse 2007, 61, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Day, M.; Wang, Z.; Ding, J.; An, X.; Ingham, C.A.; Shering, A.F.; Wokosin, D.; Ilijic, E.; Sun, Z.; Sampson, A.R.; et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci. 2006, 9, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Deutch, A.Y. Striatal plasticity in parkinsonism: Dystrophic changes in medium spiny neurons and progression in Parkinson’s disease. J. Neural. Transm. Suppl. 2006, 70, 67–70. [Google Scholar] [PubMed]
- McNeill, T.H.; Brown, S.A.; Rafols, J.A.; Shoulson, I. Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Res. 1988, 455, 148–152. [Google Scholar] [CrossRef]
- Stephens, B.; Mueller, A.J.; Shering, A.F.; Hood, S.H.; Taggart, P.; Arbuthnott, G.W.; Bell, J.E.; Kilford, L.; Kingsbury, A.E.; Daniel, S.E.; et al. Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. Neuroscience 2005, 132, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Zaja-Milatovic, S.; Milatovic, D.; Schantz, A.M.; Zhang, J.; Montine, K.S.; Samii, A.; Deutch, A.Y.; Montine, T.J. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology 2005, 64, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Nagerl, U.V.; Eberhorn, N.; Cambridge, S.B.; Bonhoeffer, T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 2004, 44, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Yuste, R.; Bonhoeffer, T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 2001, 24, 1071–1089. [Google Scholar] [CrossRef] [PubMed]
- Singleton, A.B.; Farrer, M.; Johnson, J.; Singleton, A.; Hague, S.; Kachergus, J.; Hulihan, M.; Peuralinna, T.; Dutra, A.; Nussbaum, R.; et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science 2003. [Google Scholar] [CrossRef]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Tabarean, I.; Tang, Y.; Cartier, A.; Masliah, E.; Roy, S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J. Neurosci. 2010, 30, 8083–8095. [Google Scholar] [CrossRef] [PubMed]
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 2010, 65, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, K.J.; Taylor, J.K.; Osterberg, V.R.; Churchill, M.J.; Pollock, E.; Moore, C.; Meshul, C.K.; Unni, V. Presynaptic alpha-synuclein aggregation in a mouse model of Parkinson’s disease. J. Neurosci. 2014, 34, 2037–2050. [Google Scholar] [CrossRef] [PubMed]
- Tofaris, G.K.; Garcia, R.P.; Humby, T.; Lambourne, S.L.; O’Connell, M.; Ghetti, B.; Gossage, H.; Emson, P.C.; Wilkinson, L.S.; Goedert, M.; et al. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): Implications for Lewy body disorders. J. Neurosci. 2006, 26, 3942–3950. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Reitböck, P.; Anichtchik, O.; Bellucci, A.; Iovino, M.; Ballini, C.; Fineberg, E.; Ghetti, B.; Della Corte, L.; Spano, P.; Tofaris, G.K.; et al. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 2010, 133, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Kehm, V.M.; Lee, E.B.; Soper, J.H.; Li, C.; Trojanowski, J.Q.; Lee, V.M. alpha-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies. J. Neurosci. 2011, 31, 10076–10087. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Postuma, R.B.; Bloem, B.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.M.; Hardy, J.; Lang, A.E.; et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov. Disord. 2014, 29, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Milenkovic, I.J.; Preusser, M.; Budka, H. Nigral burden of alpha-synuclein correlates with striatal dopamine deficit. Mov. Disord. 2008, 23, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.A.; Voorn, P.; Berendse, H.W.; Groenewegen, H.J.; Rozemuller, A.J.; van de Berg, W.D. Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov. Disord. 2014, 29, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Ben-Shlomo, Y.; Lees, A.J. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002, 125, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Przedborski, S. A tale on animal models of Parkinson’s disease. Mov. Disord. 2011, 26, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U.; Arbuthnott, G.W. Quantitative recording of rotational behavior in rats after 6-hydroxydopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970, 24, 485–493. [Google Scholar] [CrossRef]
- Langston, J.W.; Forno, L.S.; Rebert, C.S.; Irwin, I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey. Brain Res. 1984, 292, 390–394. [Google Scholar] [CrossRef]
- Chesselet, M.F.; Richter, F. Modelling of Parkinson’s disease in mice. Lancet Neurol. 2011, 10, 1108–1118. [Google Scholar] [CrossRef]
- Beal, M.F. Parkinson’s disease: A model dilemma. Nature 2010, 466, S8–S18. [Google Scholar] [CrossRef] [PubMed]
- Tanji, K.; Mori, F.; Mimura, J.; Ito, K.; Kaskita, A.; Takahashi, H.; Wakabayashi, K. Proteinase K-resistant α-synuclein is deposited in presynapses in human Lewy body disease and A53T α-synuclein transgenic mice. Acta Neuropathol. 2010, 120, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Neuropathology of sporadic Parkinson’s disease: Evaluation and changes of concepts. Mov. Disord. 2012, 27, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Ulane, C.M.; Burke, R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Schaeffer, W.J. A transgenic mouse model of dementia with Lewy bodies suggest a link between α-synuclein expression, presynaptic vesicle pathology and cognitive deficit. Future Neurol. 2012, 7, 13–17. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz-Schaeffer, W.J. Is Cell Death Primary or Secondary in the Pathophysiology of Idiopathic Parkinson’s Disease? Biomolecules 2015, 5, 1467-1479. https://doi.org/10.3390/biom5031467
Schulz-Schaeffer WJ. Is Cell Death Primary or Secondary in the Pathophysiology of Idiopathic Parkinson’s Disease? Biomolecules. 2015; 5(3):1467-1479. https://doi.org/10.3390/biom5031467
Chicago/Turabian StyleSchulz-Schaeffer, Walter J. 2015. "Is Cell Death Primary or Secondary in the Pathophysiology of Idiopathic Parkinson’s Disease?" Biomolecules 5, no. 3: 1467-1479. https://doi.org/10.3390/biom5031467




