Characterization of Receptor Binding Profiles of Influenza A Viruses Using An Ellipsometry-Based Label-Free Glycan Microarray Assay Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus Propagation
2.2. Virus Purification
2.3. Synthesis of Biotinylated Glycans
| Glycan I.D. | Glycan Structures | ||||
|---|---|---|---|---|---|
| OS-1 | Gal | β-Biotin | |||
| OS-2 | GalNAc | α-Biotin | |||
| OS-3 | Gal | β1-4Glc | β-Biotin | ||
| OS-4 | Gal6S | β1-4Glc | β-Biotin | ||
| OS-5 | Gal | β1-4GlcNAc | β-Biotin | ||
| OS-6 | Gal | β1-4GlcNAc6S | β-Biotin | ||
| OS-7 | Gal | β1-3GlcNAc | β-Biotin | ||
| OS-8 | Gal | β1-3GlcNAc | β1-3Galβ1-4Glc | β-Biotin | |
| OS-9 | Gal | β1-3GalNAc | β-Biotin | ||
| OS-10 | Neu5Acα2-3 | Gal | β-Biotin | ||
| OS-11 | Neu5Acα2-3 | Gal | β1-4Glc | β-Biotin | |
| OS-12 | Neu5Acα2-3 | Gal6S | β1-4Glc | β-Biotin | |
| OS-13 | Neu5Acα2-3 | Gal | β1-4GlcNAc | β-Biotin | |
| OS-14 | Neu5Acα2-3 | Gal | β1-4GlcNAc6S | β-Biotin | |
| OS-15 | Neu5Acα2-3 | Gal | β1-3GlcNAc | β-Biotin | |
| OS-16 | Neu5Acα2-3 | Gal | β1-3GlcNAc | β1-3Galβ1-4Glc | β-Biotin |
| OS-17 | Neu5Acα2-3 | Gal | β1-3GalNAc | β-Biotin | |
| OS-18 | Neu5Acα2-6 | GalNAc | α-Biotin | ||
| OS-19 | Kdnα2-6 | Gal | β1-4Glc | β-Biotin | |
| OS-20 | Neu5Gcα2-6 | Gal | β1-4Glc | β-Biotin | |
| OS-21 | Neu5Acα2-6 | Gal | β1-4Glc | β-Biotin | |
| OS-22 | Neu5Acα2-6 | Gal | β1-4GlcNAc | β-Biotin | |
| OS-23 | Neu5Acα2-6 | Gal | β1-4GlcNAc6S | β-Biotin | |
| OS-24 | Neu5Acα2-6 | Gal | β1-3GlcNAc | β-Biotin | |
2.4. Fabrication of Glycan Microarrays
2.5. Fabrication of Influenza Virus Microarrays for Monovalent Glycan-HA Affinity Assays
2.6. Virus Binding Assay on Glycan Microarrays and Label-free Detection
3. Results
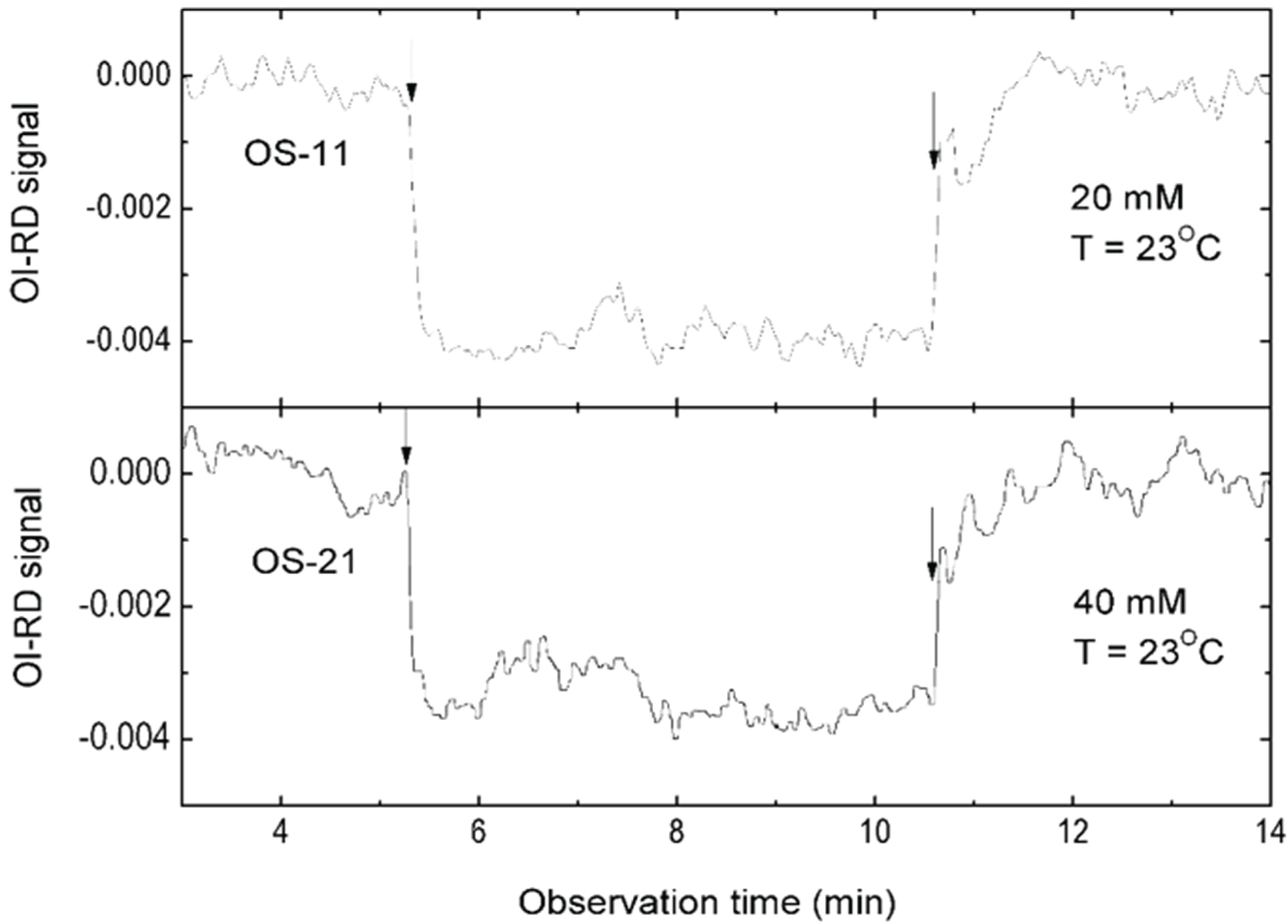
3.1. Binding Curves of Influenza A Virus to Glycan Microarrays
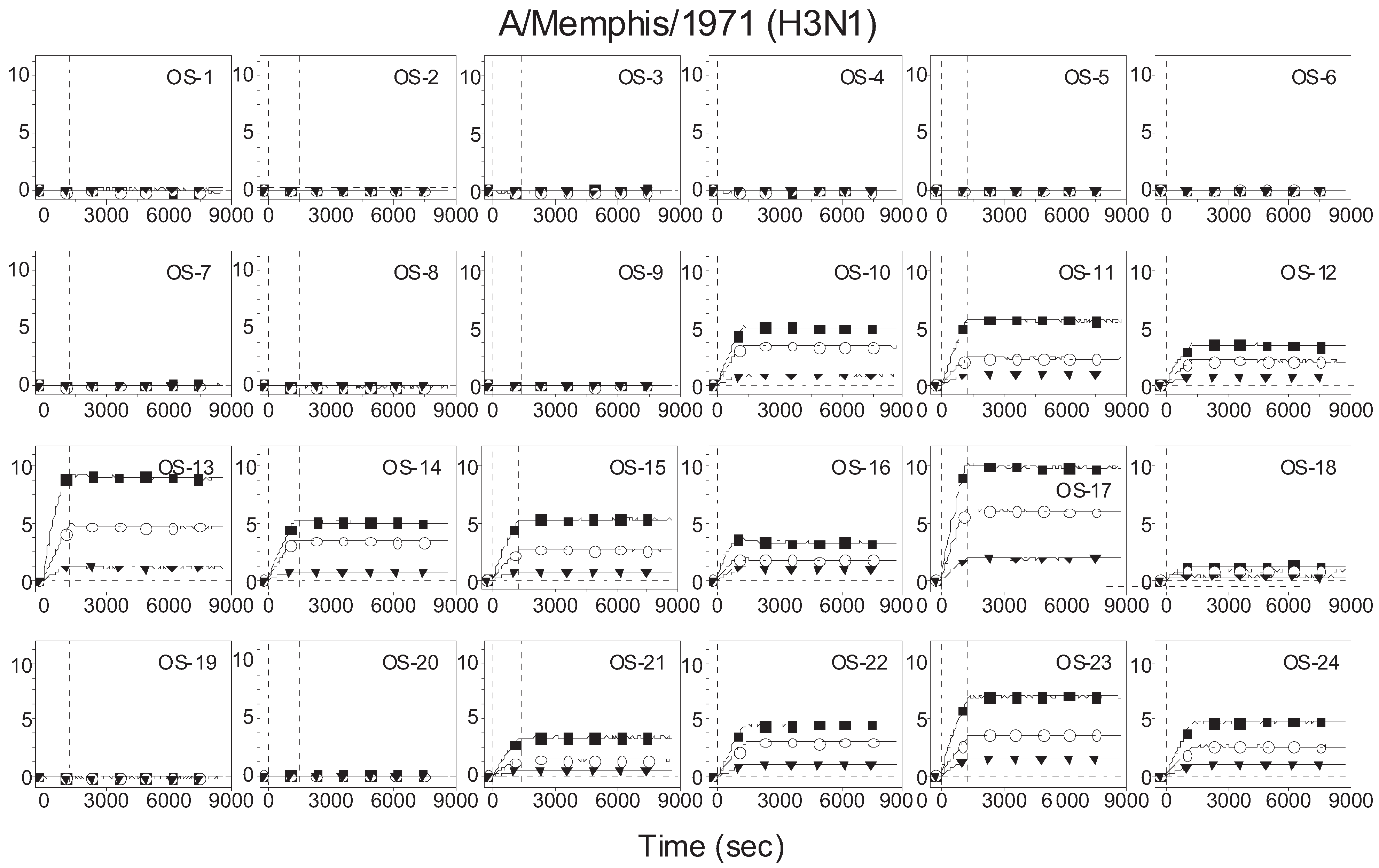
3.2. Receptor Specificity Profiles of Influenza Viruses of Subtype H3 and H1

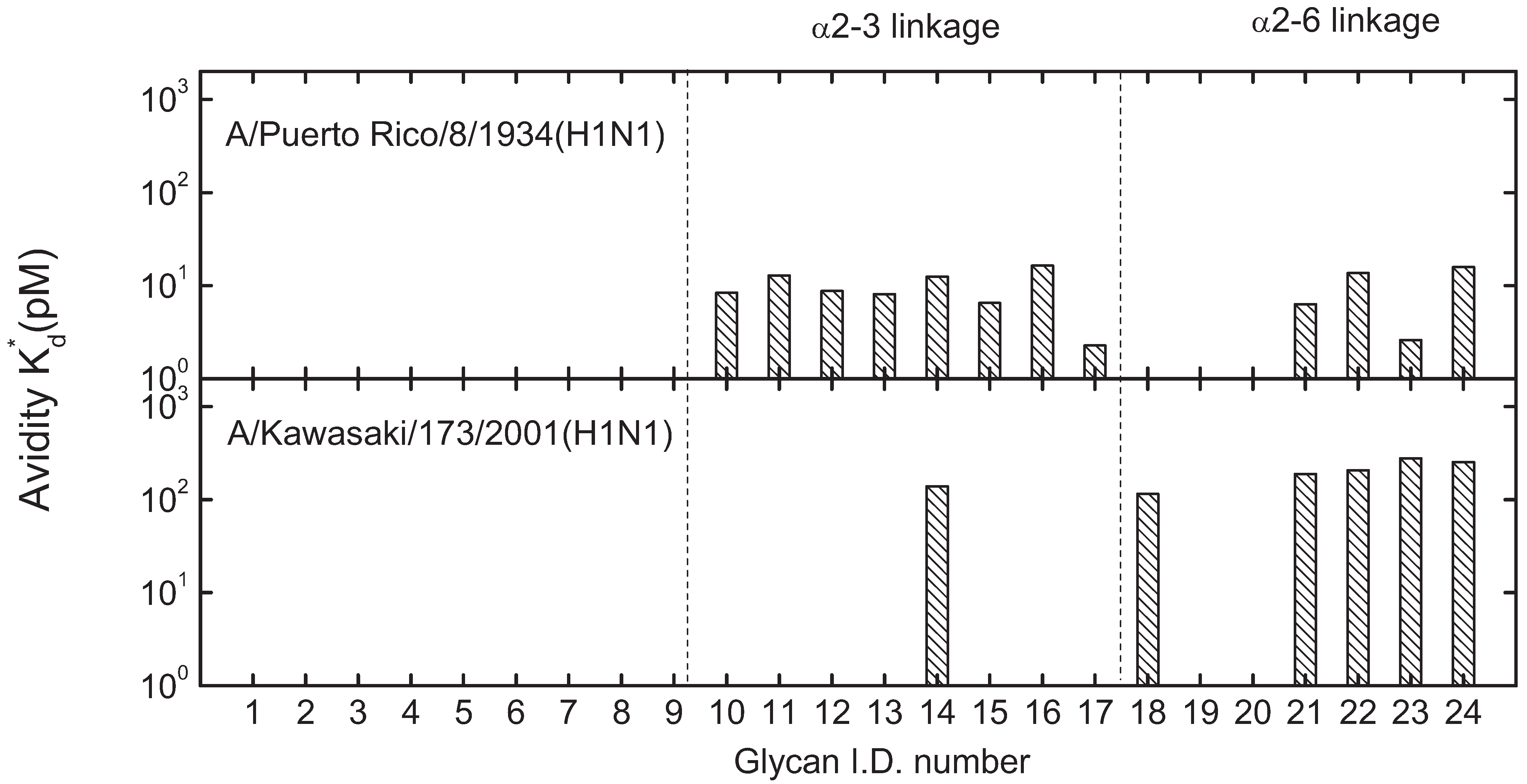
3.3. Mono-valent Equilibrium Dissociation Constants (Affinity Constants) and Thermodynamics of Sialyl Glycan-HA Reactions
| Glycans | Kinetics constants | Apparent kinetics constants | ||||
|---|---|---|---|---|---|---|
| Kon (1/M·s) | Koff (1/s) | Kd (mM) | K*on (1/M·s) | K*off (1/s) | K*d (mM) | |
| α2-3 (OS-11) | 2.8 | 7.36 × 10−2 | 26.3 | 7.2 × 103 | 4.1 × 10−6 | 5.8 × 10−7 |
| α2-6 (OS-21) | 2.7 | 1.28 × 10−1 | 47.6 | 7.0 × 103 | <3.0 × 10−6 | <4.3 × 10−7 |
| Glycans | Temperature (K) | Kd (mM) | ΔG (kcal/mol) | ΔH (kcal/mol) | ΔS (kcal/mol·K) | TΔS (kcal/mol) |
|---|---|---|---|---|---|---|
| α2-3 sialyllactose (OS-11) | 288 | 2.6 | −3.44 | −41.4 | −0.13 | −37.9 |
| 293 | 6.4 | −2.96 | −41.4 | −0.13 | −38.6 | |
| 296 | 18.8 | −2.35 | −41.4 | −0.13 | −39.0 | |
| 298 | 26.3 | −2.16 | −41.4 | −0.13 | −39.2 | |
| α2-6 sialyllactose (OS-21) | 288 | 6.8 | −2.87 | −32.2 | −0.10 | −29.2 |
| 293 | 11.2 | −2.63 | −32.2 | −0.10 | −29.7 | |
| 296 | 23.8 | −2.21 | −32.2 | −0.10 | −30.0 | |
| 298 | 47.6 | −1.82 | −32.2 | −0.10 | −30.2 |
4. Discussion and Conclusions
| Virus strain | Subtype | 138 | 190 | 225 | 226 | 228 | α2-3 | α2-6 |
|---|---|---|---|---|---|---|---|---|
| A/Kawasaki/173/2001 | H1 | S | D | D | Q | G | − | + |
| A/Puerto Rico/8/1934 | H1 | A | E | D | Q | G | + | + |
| A/Memphis/1971 | H3 | A | E | G | L | S | + | + |
| A/Udorn/307/1972 | H3 | A | E | G | L | S | + | + |
| A/Philippines/2/1982/X-79 | H3 | T | E | G | L | S | + | + |
| A/Memphis/14/1996-M [22] | H1 | S | D | D | Q | G | − | + |
| A/New Caledorial/20/1999 [57] | H1 | S | D | D | Q | G | − | + |
| A/Oklahoma/447/2008 [58] | H1 | S | D | D | Q | G | − | + |
| A/Ohio/07/2009 [57] | H1 | A | D | D | Q | G | − | + |
| A/Texas/05/2009 [57] | H1 | A | D | D | Q | G | − | + |
| A/NewYork/18/2009 [57] | H1 | A | D | D | Q | G | − | + |
| A/South Carolina/1/1918 [59] | H1 | A | D | D | Q | G | − | + |
| A/South Carolina/1/1918 [12] | H1 | A | D | D | Q | G | − | + |
| A/South Carolina/1/1918 [56] | H1 | A | D | D | Q | G | − | + |
| A/South Carolina/1/1918 (D225G) [56] | H1 | A | D | G | Q | G | + | + |
| A/California/4/2009 [56] | H1 | A | D | D | Q | G | − | + |
| A/California/4/2009 (D225E) [56] | H1 | A | D | E | Q | G | − | + |
| A/California/4/2009 (D225G) [56] | H1 | A | D | G | Q | G | + | + |
| A/California/4/2009 [22] | H1 | A | D | D | Q | G | + | + |
| A/California/07/2009 [23] | H1 | A | D | D | Q | G | + | + |
| A/California/07/2009 [60] | H1 | A | D | D | Q | G | − | + |
| A/Brisbane/59/2007 [23] | H1 | S | N | D | Q | G | + | + |
| A/Hamburg/5/2009 [22] | H1 | A | D | D | Q | G | + | + |
| A/Iowa/1/2006 [22] | H1 | A | D | N | Q | G | + | + |
| A/Mexico/Indre/4114/2009 [57] | H1 | A | D | G | Q | G | + | + |
| A/New Jersey/1976 [22] | H1 | A | D | G | Q | G | + | + |
| A/New Jersey/1976 [57] | H1 | A | D | G | Q | G | + | + |
| A/New York/1/1918 [59] | H1 | A | D | G | Q | G | + | + |
| A/New York/1/1918 [12] | H1 | A | D | G | Q | G | + | + |
| A/New York/1/1918(D190E) [12] | H1 | A | E | G | Q | G | + | − |
| A/New York/4/2009 [57] | H1 | A | D | G | Q | G | + | + |
| A/Texas/36/1991 [12] | H1 | S | D | D | Q | G | + | + |
| A/Puerto Rico/8/1934 [50] | H1 | A | E | D | Q | G | + | + |
| A/Puerto Rico/8/1934 [15] | H1 | A | E | D | Q | G | + | + |
| A/Fort Monmouth/1/1947 [15] | H1 | A | D | G | Q | G | + | + |
| A/Roma/1/1949 [15] | H1 | A | D | G | Q | G | + | + |
| A/Malaya/302/1954 [15] | H1 | A | D | G | Q | G | − | + |
| A/Denver/1957 [15] | H1 | A | E | D | Q | G | + | − |
| A/New Jersey /8/1976 [15] | H1 | A | D | G | Q | G | − | + |
| A/USSR/90/1977 [15] | H1 | S | D | G | Q | G | − | + |
| A/Brazil/11/1978 [15] | H1 | S | D | G | Q | G | − | + |
| A/India/6263/1980 [15] | H1 | S | N | D | Q | G | − | + |
| A/Chile/1/1983 [15] | H1 | A | D | N | Q | G | − | + |
| A/Taiwan/1/1986 [15] | H1 | S | D | G | Q | G | − | + |
| A/Memphis/12/1986 [15] | H1 | S | D | G | Q | G | − | + |
| A/CHR/157/1983 [15] | H1 | S | D | D | Q | G | − | + |
| A/Kawasaki/173/2001 [51] | H1 | S | D | D | Q | G | − | + |
| A/Kawasaki/173/2001 [8] | H1 | S | D | D | Q | G | − | + |
| A/Aichi/2/1968 [46] | H3 | A | E | G | L | S | + | + |
| A/Aichi/2/1968 [61] | H3 | A | E | G | L | S | + | + |
| A/Memphis/102/1972 [46] | H3 | A | E | G | L | S | + | + |
| A/LosAngeles/2/1987 [46] | H3 | A | E | G | L | S | + | + |
| A/Udorn/307/1972 [61] | H3 | A | E | G | L | S | + | + |
| A/Philippines/2/1982/X-79 [17] | H3 | A | E | G | I | S | + | + |
| A/Victoria/3/1975 [61] | H3 | A | E | G | L | S | + | + |
| A/Shanghai/11/1989 [46] | H3 | A | E | G | L | S | + | + |
| A/Udorn/307/1972 [46] | H3 | A | E | G | L | S | + | + |
| A/Udorn/307/1972 [17] | H3 | A | E | G | L | S | + | + |
| A/Udorn/307/1972(E190D) [45] | H3 | A | D | G | L | S | − | + |
| A/Hongkong/1/1968 [60] | H3 | A | E | G | L | S | − | + |
| A/Texas/1/1977 [61] | H3 | A | E | G | L | S | − | + |
| A/Shanghai/31/1980 [61] | H3 | A | E | G | L | S | − | + |
| A/LosAngeles/2/1987 [61] | H3 | A | E | G | L | S | − | + |
| A/Oklahoma/483/2008 [58] | H3 | A | D | D | I | S | − | + |
| A/Oklahoma/323/2003 [17] | H3 | A | D | D | I | S | − | + |
| A/Oklahoma/369/2005 [17] | H3 | S | D | D | I | S | − | + |
| A/Oklahoma/1992/2005 [17] | H3 | A | D | D | I | S | − | + |
| A/Tottori/872K4/1994 [62] | H3 | A | D | G | L | S | − | + |
| A/Tottori/872AM2/1994 [62] | H3 | A | D | G | L | S | − | + |
| A/Tottori/872AM4/1994 [62] | H3 | A | D | G | Q | S | + | − |
| A/Tottori/872AM1AL3/1994 [62] | H3 | A | D | G | Q | S | + | − |
| A/Tottori/872AM2AL3/1994 [62] | H3 | A | D | G | Q | S | + | − |
| A/Mem/1/71-Bel42 [47] | H3 | A | E | G | L | S | + | + |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Horimoto, T.; Kawaoka, Y. Pandemic threat posed by avian influenza A viruses. Clin. Microbiol. Rev. 2001, 14, 129–149. [Google Scholar] [CrossRef] [PubMed]
- Molinari, N.A.M.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 2007, 25, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [PubMed]
- Taubenberger, J.K.; Morens, D.M. Pandemic influenza—Including a risk assessment of H5N1. Rev. Sci. Tech. 2009, 28, 187–202. [Google Scholar] [PubMed]
- Morens, D.M.; Fauci, A.S. The 1918 influenza pandemic: Insights for the 21st century. J. Infect. Dis. 2007, 195, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. The persistent legacy of the 1918 influenza virus. N. Engl. J. Med. 2009, 361, 225–229. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Ng, K.H.L.; Que, T.-L.; Chan, J.M.C.; Tsang, K.-Y.; Tsang, A.K.L.; Chen, H.; Yuen, K.-Y. Avian influenza A H5N1 virus: A continuous threat to humans. Emerg. Microbes Infect. 2012. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kiso, M.; Fukuyama, S.; Nakajima, N.; Imai, M.; Yamada, S.; Murakami, S.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Sakoda, Y.; et al. Characterization of H7N9 influenza A viruses isolated from humans. Nature 2013, 501, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Kawaoka, Y. Flu transmission work is urgent. Nature 2012, 482, 155–155. [Google Scholar] [CrossRef] [PubMed]
- Herfst, S.; Schrauwen, E.J.A.; Linster, M.; Chutinimitkul, S.; de Wit, E.; Munster, V.J.; Sorrell, E.M.; Bestebroer, T.M.; Burke, D.F.; Smith, D.J.; et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012, 336, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Kawaoka, Y. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr. Opin. Virol. 2012, 2, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Blixt, O.; Glaser, L.; Taubenberger, J.K.; Palese, P.; Paulson, J.C.; Wilson, I.A. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J. Mol. Biol. 2006, 355, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Skehel, J.J.; Wiley, D.C. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu. Rev. Biochem. 2000, 69, 531–569. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.C.; Skehel, J.J. The structure and function of the hemagglutinin membrane glycoprotein of influenza-virus. Annu. Rev. Biochem. 1987, 56, 365–394. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.N.; Dsouza, B.L. Receptor-binding properties of human and animal H1-influenza virus isolates. Virology 1989, 173, 317–322. [Google Scholar] [CrossRef]
- Connor, R.J.; Kawaoka, Y.; Webster, R.G.; Paulson, J.C. Receptor specificity in human, avian, and equine H2 and H3 influenza-virus isolates. Virology 1994, 205, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Gulati, S.; Smith, D.F.; Gulati, U.; Cummings, R.D.; Air, G.M. Receptor binding specificity of recent human H3N2 influenza viruses. Virol. J. 2007. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.N.; Paulson, J.C.; Daniels, R.S.; Skehel, J.J.; Wilson, I.A.; Wiley, D.C. Single amino-acid substitutions in influenza hemagglutinin change receptor-binding specificity. Nature 1983, 304, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Gambaryan, A.S.; Matrosovich, M.N. A solid-phase enzyme-linked assay for influenza-virus receptor-binding activity. J. Virol. Methods 1992, 39, 111–123. [Google Scholar] [CrossRef]
- Gambaryan, A.; Tuzikov, A.; Pazynina, G.; Bovin, N.; Balish, A.; Klimov, A. Evolution of the receptor binding phenotype of influenza A (H5) viruses. Virology 2006, 344, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ibrahim, M.S.; Ellakany, H.F.; Kawashita, N.; Mizuike, R.; Hiramatsu, H.; Sriwilaijaroen, N.; Takagi, T.; Suzuki, Y.; Ikuta, K. Acquisition of human-type receptor binding specificity by new H5N1 influenza virus sublineages during their emergence in birds in Egypt. PLoS Pathog. 2011, 7, e1002068. [Google Scholar] [CrossRef] [PubMed]
- Childs, R.A.; Palma, A.S.; Wharton, S.; Matrosovich, T.; Liu, Y.; Chai, W.G.; Campanero-Rhodes, M.A.; Zhang, Y.B.; Eickmann, M.; Kiso, M.; et al. Receptor-binding specificity of pandemic influenza A (H1N1) 2009 virus determined by carbohydrate microarray. Nat. Biotechnol. 2009, 27, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.Y.; Hsu, C.H.; Wang, S.C.; Liang, C.H.; Yen, H.Y.; Su, C.Y.; Chen, C.H.; Jan, J.T.; Ren, C.T.; Cheng, T.J.R.; et al. Differential receptor binding affinities of influenza hemagglutinins on glycan arrays. J. Am. Chem. Soc. 2010, 132, 14849–14856. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.C.; Blixt, O.; Collins, B.E. Sweet spots in functional glycomics. Nat. Chem. Biol. 2006, 2, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.H.; Wu, C.Y.; Greenberg, W.A.; Wong, C.H. Glycan arrays: Biological and medical applications. Curr. Opin. Chem. Biol. 2008, 12, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.P.; Fei, Y.Y.; Zhu, X.D.; Ke, Y.H.; Yu, G.L.; Lee, P. Discovering small molecule ligands of vascular endothelial growth factor that block VEGF-KDR binding using label-free microarray-based assays. Assay Drug Dev. Technol. 2013, 11, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.P.; Fei, Y.Y.; Zhu, X.D. Simultaneous measurement of 10,000 protein-ligand affinity constants using microarray-based kinetic constant assays. Assay Drug Dev. Technol. 2012, 10, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.Y.; Schmidt, A.; Bylund, G.; Johansson, D.X.; Henriksson, S.; Lebrilla, C.; Solnick, J.V.; Boren, T.; Zhu, X.D. Use of real-time, label-free analysis in revealing low-affinity binding to blood group antigens by Helicobacter pylori. Anal. Chem. 2011, 83, 6336–6341. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.Y.; Sun, Y.S.; Li, Y.H.; Lau, K.; Yu, H.; Chokhawala, H.A.; Huang, S.; Landry, J.P.; Chen, X.; Zhu, X.D. Fluorescent labeling agents change binding profiles of glycan-binding proteins. Mol. Biosyst. 2011, 7, 3343–3352. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.P.; Sun, Y.S.; Guo, X.W.; Zhu, X.D. Protein reactions with surface-bound molecular targets detected by oblique-incidence reflectivity difference microscopes. Appl. Opt. 2008, 47, 3275–3288. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, S.; Hinou, H.; Ebihara, D.; Sato, R.; Kuroiwa, S.; Nakanishi, T.; Nishimura, S.I.; Osaka, T. Attomolar detection of influenza A virus hemagglutinin human H1 and avian H5 using glycan-blotted field effect transistor biosensor. Anal. Chem. 2013, 85, 5641–5644. [Google Scholar] [CrossRef] [PubMed]
- Hushegyi, A.; Bertok, T.; Damborsky, P.; Katrlik, J.; Tkac, J. An ultrasensitive impedimetric glycan biosensor with controlled glycan density for detection of lectins and influenza hemagglutinins. Chem. Commun. 2015, 51, 7474–7477. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Cardona, C.J.; Xing, Z.; Woolcock, P.R. Genetic and phenotypic characterization of a low-pathogenicity avian influenza H11N9 virus. Arch. Virol. 2008, 153, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chokhawala, H.; Karpel, R.; Wu, B.Y.; Zhang, J.B.; Zhang, Y.X.; Jia, Q.; Chen, X. A multifunctional pasteurella multocida sialyltransferase: A powerful tool for the synthesis of sialoside libraries. J. Am. Chem. Soc. 2005, 127, 17618–17619. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chokhawala, H.A.; Huang, S.S.; Chen, X. One-pot three-enzyme chemoenzymatic approach to the synthesis of sialosides containing natural and non-natural functionalities. Nat. Protoc. 2006, 1, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, S.S.; Chokhawala, H.; Sun, M.C.; Zheng, H.J.; Chen, X. Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural α-2,6-linked sialosides: A P. Damsela α-2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew. Chem. Int. Ed. Edit. 2006, 45, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Karpel, R.; Chen, X. Chemoenzymatic synthesis of CMP-sialic acid derivatives by a one-pot two-enzyme system: Comparison of substrate flexibility of three microbial CMP-sialic acid synthetases. Bioorgan. Med. Chem. 2004, 12, 6427–6435. [Google Scholar] [CrossRef] [PubMed]
- Chokhawala, H.A.; Huang, S.S.; Lau, K.; Yu, H.; Cheng, J.S.; Thon, V.; Hurtado-Ziola, N.; Guerrero, J.A.; Varki, A.; Chen, X. Combinatorial chemoenzymatic synthesis and high-through put screening of sialosides. ACS Chem. Biol. 2008, 3, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.Y.; Landry, J.P.; Li, Y.H.; Yu, H.; Lau, K.; Huang, S.S.; Chokhawala, H.A.; Chen, X.; Zhu, X.D. An optics-based variable-temperature assay system for characterizing thermodynamics of biomolecular reactions on solid support. Rev. Sci. Instrum. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kuwert, E.; Wiktor, T.J.; Sokol, F.; Koprowsk, H. Hemagglutination by rabies virus. J. Virol. 1968, 2, 1381–1392. [Google Scholar] [PubMed]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Edit. 1998, 37, 2755–2794. [Google Scholar] [CrossRef]
- Pluckthun, A.; Pack, P. New protein engineering approaches to multivalent and bispecific antibody fragments. Immunotechnology 1997, 3, 83–105. [Google Scholar] [CrossRef]
- Li, Y.; Bostick, D.L.; Sullivan, C.B.; Myers, J.L.; Griesemer, S.B.; StGeorge, K.; Plotkin, J.B.; Hensley, S.E. Single hemagglutinin mutations that alter both antigenicity and receptor binding avidity influence influenza virus antigenic clustering. J. Virol. 2013, 87, 9904–9910. [Google Scholar] [CrossRef] [PubMed]
- Hensley, S.E.; Das, S.R.; Bailey, A.L.; Schmidt, L.M.; Hickman, H.D.; Jayaraman, A.; Viswanathan, K.; Raman, R.; Sasisekharan, R.; Bennink, J.R.; et al. Hemagglutinin receptor binding avidity drives influenza A virus antigenic drift. Science 2009, 326, 734–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pekosz, A.; Newby, C.; Bose, P.S.; Lutz, A. Sialic acid recognition is a key determinant of influenza A virus tropism in murine trachea epithelial cell cultures. Virology 2009, 386, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Matrosovich, M.; Tuzikov, A.; Bovin, N.; Gambaryan, A.; Klimov, A.; Castrucci, M.R.; Donatelli, I.; Kawaoka, Y. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 2000, 74, 8502–8512. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kato, H.; Naeve, C.W.; Webster, R.G. Single-amino-acid substitution in an antigenic site of influenza-virus hemagglutinin can alter the specificity of binding to cell membrane-associated gangliosides. J. Virol. 1989, 63, 4298–4302. [Google Scholar] [PubMed]
- Suzuki, Y.; Ito, T.; Suzuki, T.; Holland, R.E.; Chambers, T.M.; Kiso, M.; Ishida, H.; Kawaoka, Y. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 2000, 74, 11825–11831. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.N.; Paulson, J.C. Receptor determinants of human and animal influenza-virus isolates—Differences in receptor specificity of the hemagglutinin-H3 based on species of origin. Virology 1983, 127, 361–373. [Google Scholar] [CrossRef]
- Blixt, O.; Head, S.; Mondala, T.; Scanlan, C.; Huflejt, M.E.; Alvarez, R.; Bryan, M.C.; Fazio, F.; Calarese, D.; Stevens, J.; et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 17033–17038. [Google Scholar] [CrossRef] [PubMed]
- Shinya, K.; Ebina, M.; Yamada, S.; Ono, M.; Kasai, N.; Kawaoka, Y. Avian flu: Influenza virus receptors in the human airway. Nature 2006, 440, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S. Label-free Detection of Biomolecular Interactions in Microarray Format Using Oblique-incidence Reflectivity Difference Microscopes. Ph.D. Thesis, University of California, Davis, CA, USA, 2010. [Google Scholar]
- Sauter, N.K.; Bednarski, M.D.; Wurzburg, B.A.; Hanson, J.E.; Whitesides, G.M.; Skehel, J.J.; Wiley, D.C. Hemagglutinins from two influenza-virus variants bind to sialic-acid derivatives with millimolar dissociation-constants—A 500-MHz proton nuclear magnetic-resonance study. Biochemistry 1989, 28, 8388–8396. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.E.; Sauter, N.K.; Skehel, J.J.; Wiley, D.C. Proton nuclear-magnetic-resonance studies of the binding of sialosides to intact influenza-virus. Virology 1992, 189, 525–533. [Google Scholar] [CrossRef]
- Dam, T.K.; Brewer, C.F. Thermodynamic studies of lectin-carbohydrate interactions by isothermal titration calorimetry. Chem. Rev. 2002, 102, 387–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, Y.; Qi, J.; Gao, F.; Li, Q.; Fan, Z.; Yan, J.; Gao, G.F. Molecular basis of the receptor binding specificity switch of the hemagglutinins from both the 1918 and 2009 pandemic influenza A viruses by a D225G substitution. J. Virol. 2013, 87, 5949–5958. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Rivailler, P.; Hossain, J.; Carney, P.; Balish, A.; Perry, I.; Davis, C.T.; Garten, R.; Shu, B.; Xu, X.; et al. Receptor specificity of subtype H1 influenza A viruses isolated from swine and humans in the United States. Virology 2011, 412, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yu, H.; Chen, X.; Lasanajak, Y.; Tappert, M.M.; Air, G.M.; Tiwari, V.K.; Cao, H.; Chokhawala, H.A.; Zheng, H.; et al. A sialylated glycan microarray reveals novel interactions of modified sialic acids with proteins and viruses. J. Biol. Chem. 2011, 286, 31610–31622. [Google Scholar] [CrossRef] [PubMed]
- Tumpey, T.M.; Maines, T.R.; van Hoeven, N.; Glaser, L.; Solorzano, A.; Pappas, C.; Cox, N.J.; Swayne, D.E.; Palese, P.; Katz, J.M.; et al. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 2007, 315, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Carney, P.J.; Chang, J.C.; Villanueva, J.M.; Stevens, J. Structural analysis of the hemagglutinin from the recent 2013 H7N9 influenza virus. J. Virol. 2013, 87, 12433–12446. [Google Scholar] [CrossRef] [PubMed]
- Ryan-Poirier, K.; Suzuki, Y.; Bean, W.J.; Kobasa, D.; Takada, A.; Ito, T.; Kawaoka, Y. Changes in H3 influenza A virus receptor specificity during replication in humans. Virus Res. 1998, 56, 169–176. [Google Scholar] [CrossRef]
- Xu, R.; de Vries, R.P.; Zhu, X.; Nycholat, C.M.; McBride, R.; Yu, W.; Paulson, J.C.; Wilson, I.A. Preferential recognition of avian-like receptors in human influenza A H7N9 viruses. Science 2013, 342, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, Y.; Sun, Y.-S.; Li, Y.; Yu, H.; Lau, K.; Landry, J.P.; Luo, Z.; Baumgarth, N.; Chen, X.; Zhu, X. Characterization of Receptor Binding Profiles of Influenza A Viruses Using An Ellipsometry-Based Label-Free Glycan Microarray Assay Platform. Biomolecules 2015, 5, 1480-1498. https://doi.org/10.3390/biom5031480
Fei Y, Sun Y-S, Li Y, Yu H, Lau K, Landry JP, Luo Z, Baumgarth N, Chen X, Zhu X. Characterization of Receptor Binding Profiles of Influenza A Viruses Using An Ellipsometry-Based Label-Free Glycan Microarray Assay Platform. Biomolecules. 2015; 5(3):1480-1498. https://doi.org/10.3390/biom5031480
Chicago/Turabian StyleFei, Yiyan, Yung-Shin Sun, Yanhong Li, Hai Yu, Kam Lau, James P. Landry, Zeng Luo, Nicole Baumgarth, Xi Chen, and Xiangdong Zhu. 2015. "Characterization of Receptor Binding Profiles of Influenza A Viruses Using An Ellipsometry-Based Label-Free Glycan Microarray Assay Platform" Biomolecules 5, no. 3: 1480-1498. https://doi.org/10.3390/biom5031480
APA StyleFei, Y., Sun, Y.-S., Li, Y., Yu, H., Lau, K., Landry, J. P., Luo, Z., Baumgarth, N., Chen, X., & Zhu, X. (2015). Characterization of Receptor Binding Profiles of Influenza A Viruses Using An Ellipsometry-Based Label-Free Glycan Microarray Assay Platform. Biomolecules, 5(3), 1480-1498. https://doi.org/10.3390/biom5031480





