Skin Whitening Cosmetics: Feedback and Challenges in the Development of Natural Skin Lighteners
Abstract
:1. Introduction
1.1. Why Such a Practice?
1.2. Melanogenesis
1.2.1. Mechanism
- In the absence of cysteine or gluthatione, one can observe the non-enzymatic cyclisation of l-DOPA into leucoDOPAchrome. This compound is further oxidized into dopachrome, the precursor of dihydroindole (DHI) and dihydroindole-2-carboxylic acid (DHICA), which leads through a series of oxidation reactions to the synthesis of UV-protective and ROS-scavenger eumelanins, which are brown or black pigments [11,60,61,62].
1.2.2. Multidirectional Approaches to Modulating Skin Pigmentation
- modulators of tyrosinase glycosylation and maturation or acceleration of its degradation [11],
- inhibitors of the α-MSH (α-melanocyte-stimulating hormone)/cAMP (cyclic adenosine monophosphate)-dependent signaling pathway and the subsequent α-MSH-induced melanin production [53],
- modulators of the mitogen-activated protein kinases (MAPK) signaling pathway [53],
- modulators of the Wnt signaling pathway [53],
- inhibitors of the NO (nitric oxide) signaling pathway [53],
- ATP7A (also known as Menkes’ protein or MNK) trafficking inhibitors [53],
- down-regulators of MC1R (melanocortin 1 receptor) activity [19],
- inducers of autophagy, a cellular degradation process that affects skin color by regulation of melanin degradation in normal human epidermal keratinocytes [74].
1.2.3. Testing the Whitening Potential of a Given Substance
- in vitro assays constitute the first method to rapidly identify individual components or potentially active extracts. They are used to evaluate the tyrosinase or TRP-2 inhibition potency of single molecules or natural extracts.In vitro screening is usually performed using mushroom tyrosinase, generally purified from Agaricus bisporus (cheap and easily available) according to a protocol adapted from methods described earlier [75,76,77]; extrapolation to humans might be difficult. Only a few bioassays were actually performed using monomeric human tyrosinase, which is hard to purify as it is membrane-bound rather than cytosolic like its tetrameric mushroom counterpart [78]; also commercially available, it is seldom used as it remains quite expensive. However, the use of mammalian tyrosinase should be considered rather than the mushroom one for in vitro assays, as the inhibitors’ affinity for the mammalian one is generally lower than for the mushroom one [79]. Hence, numerous “false positives,” e.g., extracts or single molecules that are active inhibitors of mushroom tyrosinase but are inefficient once in contact with mammalian tyrosinase, might be avoided [23,80]. Recently, some studies were nevertheless performed using crude extracts of human melanocytes as the enzyme source [11].Tyrosinase activity is determined spectrophotometrically: the increase in absorption due to the DOPAchrome formation is recorded at 475–480 nm as a function of time. High-throughput screening can be performed using in vitro protocols at a reasonable cost as the assays can be realized in 96-well plates and the procedure can be totally automated. The results are either expressed as inhibition percentages or as inhibition concentration (IC50), in comparison with a positive control, generally kojic acid, but also Glycyrrhiza glabra or Morus alba extracts. The notion of “Relative Inhibitory Activity” (RA) has been introduced recently to facilitate the direct comparison of inhibitors described in various studies. RA is obtained by dividing the IC50 of the positive control by that of the inhibitor of interest [11].The DOPAchrome tautomerase, also known as tyrosinase-related protein 2 (TRP-2), presents a cellular distribution in the melanocytes quite similar to tyrosinase [81]. This enzyme is strongly involved in the regulation of the eumelanin synthesis, a late step in melanogenesis [82]. Some inhibitors have already been identified, e.g., N-(3,5-dimethylphenyl)-3-methoxybenzamide [83] and Neolitsea aciculata extract [84]. Further identification of TRP-2 inhibitors appears to be crucial, and a bioassay consisting of spectrophotometrical monitoring at 308 nm of the absorbance increase due to the TRP-2 controlled tautomerization of DOPAchrome to DHICA as a function of time has been developed [85].
- in cellulo and ex vivo assays: The whitening potency of a substance of interest can be appraised by the spectrophotometrical monitoring of the intracellular tyrosinase activity or of the intracellular melanin production after cell extraction. Several protocols have been developed depending on the cell lineage employed, the culture conditions, and the method employed to evaluate the inhibition activity [86,87,88].The evaluation of cellular MITF expression enables the identification of whitening substances that do not, or only at a very low level, display tyrosinase activity [69,89].Cultures of melanocytes may be used to assess the whitening properties of single molecules or natural extracts as they closely mimic physiologic conditions. They enable the study of the global effect of such agents on the melanin synthesis in melanocytes. However, melanocytes are difficult to maintain in culture. Hence this method, being complex and expensive, is usually not appropriate to confirm the activity of compounds the whitening activity of which was already assessed in vitro.Cultures of B16 melanoma cells, models for human skin cancers, are frequently used for the study of whitening potency [78]. However, one should bear in mind that cancerous cell lines display, owing to their nature, several abnormal functions and subsequently do not accurately mimic reality.Co-cultures of melanocytes and keratinocytes even more narrowly reproduce the in vivo situation and enable us to have a closer look at the interaction between both types of cells in the melanization epidermal unit and at the melanosomes transfer [90]. However, these systems are expensive and their implementation is difficult.
- in vivo assays and clinical trials: Mammalian skin is generally preferred to evaluate the efficacy and innocuousness of a given substance [93]: several animal models, more reliable than in vitro tests, have been used, e.g., the mouse [92,94], the zebrafish [94,95,96], the guinea pig [97,98], and the Yucatan swine [99,100]. The zebrafish presents several advantages, including easy maintenance and handling of the animals, short generation times, and high efficiency of drug penetration through the skin [96,101,102]. Relatively small, easily maintained, and displaying rather short generation times, mice are used to more closely approximate human reactions as their skin is more comparable to human skin than that of zebrafish [102]. Shaved mice present even higher drug penetration compared to non-shaved ones [102]. In contrast to mice, the epidermis of guinea pigs displays a moderate number of melanocytes and melanosomes distributed in a similar way to human skin [103]. Given the close morphologic and functional similarities between pig and human skins (similar epidermis thickness, similar epidermal cells turnover time, etc.), the effectiveness of depigmenting agents was also often evaluated in Yucatan miniature swine [99,100,104,105,106]. More complete studies taking into account the quantification of melanin production, the evaluation of the expression of cellular factors and tyrosinase, etc., may thus be undertaken in such robust integrative experimental models. It is important to remember that experimentation on animals to test cosmetic ingredients and finished cosmetics has been banned in the EU as well as in numerous countries throughout the world [23]. On the contrary, animal experimentation is still practiced by the pharmaceutical industry: dermatological whitening agents delivered only under medical prescription are tested for safety, efficacy, and liability on animal models before they are considered for widespread human use.
1.3. Traditional Whitening Products
1.4. Current Regulations
2. Development of Natural Whitening Ingredients
2.1. State of the Art
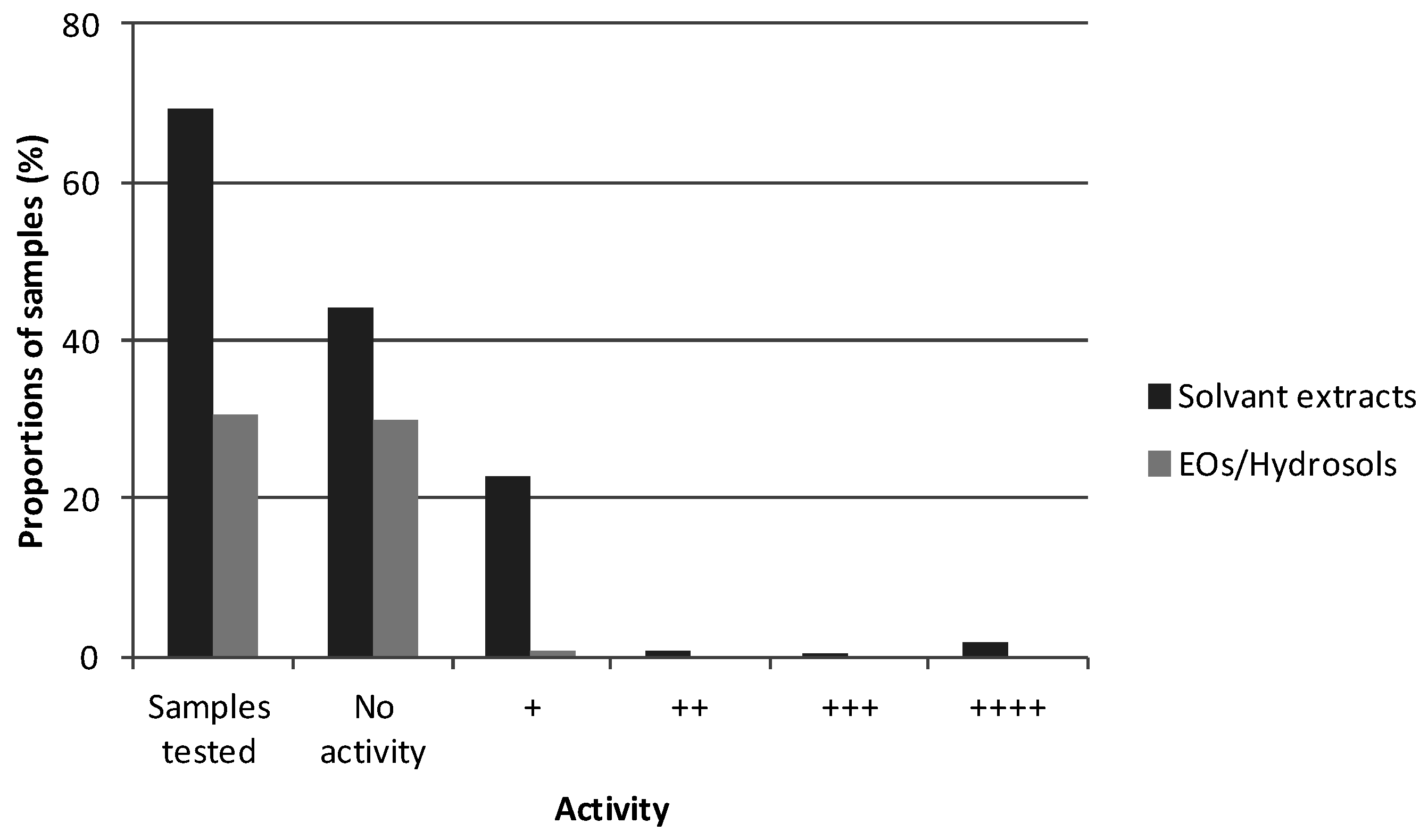
2.2. Lab Reality: Our Experience
2.3. Further Development of a Cosmetic Ingredient
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Naidoo, L.; Khoza, N.; Dlova, N.C. A fairer face, a fairer tomorrow? A review of skin lighteners. Cosmetics 2016, 3, 33. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Grimes, P.; Nordlund, J.J.; Pandya, A.G.; Taylor, S.; Rendon, M.; Ortonne, J.-P. Increasing our understanding of pigmentary disorders. J. Am. Acad. Dermatol. 2006, 54, S255–S261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gao, J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J. Investig. Dermatol. 2008, 13, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-H.; Ding, H.-Y.; Hung, W.J.; Liang, C.-H. Antioxidative characteristics and inhibition of alpha-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from Origanum vulgare. Exp. Dermatol. 2010, 19, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Kim, Y.J. Piceatannol inhibits melanogenesis by its antioxidative actions. Biol. Pharm. Bull. 2007, 30, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, W.J.; Chang, S.E.; Lee, G.-Y. Hesperidin, a popular antioxidant inhibits melanogenesis via Erk1/2 mediated MITF degradation. Int. J. Mol. Sci. 2015, 16, 18384–18395. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Kishimoto, N.; Kakino, Y.; Mochida, K.; Fujita, T. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J. Agric. Food Chem. 2004, 52, 4893–4898. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Li, W.; Huang, Y.; Chen, Y.; Jin, B.; Chen, N.; Ding, Z.; Ding, X. Antioxidant, antityrosinase and antitumor activity comparison: The potential utilization of fibrous root part of Bletilla striata (Thunb.) Reichb.f. PLoS ONE 2013, 8, e58004. [Google Scholar] [CrossRef] [PubMed]
- Kerdudo, A.; Burger, P.; Merck, F.; Dingas, A.; Rolland, Y.; Michel, T.; Fernandez, X. Development of a natural ingredient—Natural preservative: A case study. C. R. Chim. 2016, 19, 1077–1089. [Google Scholar] [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Nishida, J.; Saito, S.; Kawabata, J. Inhibitory effects of 5,6,7-trihydroxyflavones on tyrosinase. Molecules 2007, 12, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Hirata, N.; Masuda, M.; Naruto, S.; Murata, K.; Wakabayashi, K.; Matsuda, H. Inhibitory effects of Citrus hassaku extract and its flavanone glycosides on melanogenesis. Biol. Pharm. Bull. 2009, 32, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents—Existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Li, E.P.H.; Min, H.J.; Belk, R.W.; Kimura, J.; Bahl, S. Skin lightening and beauty in four Asian cultures. Adv. Consum. Res. 2008, 35, 444–449. [Google Scholar]
- Tsuchiya, T.; Yamada, K.; Minoura, K.; Miyamoto, K.; Usami, Y.; Kobayashi, T.; Hamada-Sato, N.; Imada, C.; Tsujibo, H. Purification and determination of the chemical structure of the tyrosinase inhibitor produced by Trichoderma viride strain H1-7 from a marine environment. Biol. Pharm. Bull. 2008, 31, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Kamakshi, R. Fairness via formulations: A review of cosmetic skin-lightening ingredients. J. Cosmet. Sci. 2012, 63, 43–54. [Google Scholar] [PubMed]
- Ashikari, M. Cultivating Japanese whiteness: The “whitening” cosmetics boom and the Japanese identity. J. Mater. Cult. 2005, 10, 73–91. [Google Scholar] [CrossRef]
- Chaudhri, S.K.; Jain, N.K. History of cosmetics. Asian J. Pharm. 2009, 3, 164. [Google Scholar]
- Hall, R.E. The bleaching syndrome: Western civilization vis-à-vis inferiorized people of color. In The Melanin Millennium; Hall, R.E., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 1–18. [Google Scholar]
- Fernandez, X.; Michel, T.; Azoulay, S. Actifs cosmétiques à effet blanchissant—Nature, efficacité et risques. Tech. Ing. 2015, J2300, 33. (In French) [Google Scholar]
- Porta, E.A. Pigments in aging: An overview. Ann. N. Y. Acad. Sci. 2002, 959, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Rendon, M.; Berneburg, M.; Arellano, I.; Picardo, M. Treatment of melasma. J. Am. Acad. Dermatol. 2006, 54, S272–S281. [Google Scholar] [CrossRef] [PubMed]
- James, W.D.; Berger, T.; Elston, D. Andrews’ Diseases of the Skin: Clinical Dermatology, 12th ed.; Elsevier: Philadelphia, PA, USA, 2015. [Google Scholar]
- Tunzi, M.; Gray, G.R. Common skin conditions during pregnancy. Am. Fam. Physician 2007, 75, 211–218. [Google Scholar] [PubMed]
- Ezzedine, K.; Sheth, V.; Rodrigues, M.; Eleftheriadou, V.; Harris, J.E.; Hamzavi, I.H.; Pandya, A.G. Vitiligo is not a cosmetic disease. J. Am. Acad. Dermatol. 2015, 73, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G.; Greco, A.; Didona, D.; Didona, B.; Granata, G.; Manno, A.; Pasquariello, B.; Magliulo, G. Vitiligo: Pathogenesis, clinical variants and treatment approaches. Autoimmun. Rev. 2016, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- McDougall, A. Skin Whitening Products Have Global Potential IF Marketed Correctly. Available online: http://www.cosmeticsdesign-asia.com/Market-Trends/Skin-whitening-products-have-global-potential-IF-marketed-correctly (accessed on 20 September 2016).
- McDougall, A. Skin Lightening Trend in Asia Boosts Global Market. Available online: http://www.cosmeticsdesign-asia.com/Market-Trends/Skin-lightening-trend-in-Asia-boosts-global-market (accessed on 20 September 2016).
- Desmedt, B.; Courselle, P.; De Beer, J.O.; Rogiers, V.; Grosber, M.; Deconinck, E.; De Paepe, K. Overview of skin whitening agents with an insight into the illegal cosmetic market in Europe. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Kpanake, L.; Mullet, E. Motives for skin bleaching among West Africans. Househ. Pers. Care Today 2011, 6, 6–9. [Google Scholar]
- Hall, R.E. The Melanin Millennium: Skin Color as 21st Century International Discourse; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- De Souza, M.M. The concept of skin bleaching in Africa and its devastating health implications. Clin. Dermatol. 2008, 26, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Raper, H.S. The anaerobic oxidases. Physiol. Rev. 1928, 8, 245–282. [Google Scholar]
- Mason, H.S. The chemistry of melanin. III. Mechanism of the oxidation of trihydroxyphenylalanine by tyrosinase. J. Biol. Chem. 1948, 172, 83–99. [Google Scholar] [PubMed]
- Cooksey, C.J.; Garratt, P.J.; Land, E.J.; Pavel, S.; Ramsden, C.A.; Riley, P.A.; Smit, N.P. Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J. Biol. Chem. 1997, 272, 26226–26235. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Kothari, S.; Chavan, B.; Spencer, J.D. Regulation of melanogenesis—Controversies and new concepts. Exp. Dermatol. 2008, 17, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Seiji, M.; Fitzpatrick, T.B.; Birbeck, M.S. The melanosome: A distinctive subcellular particle of mammalian melanocytes and the site of melanogenesis. J. Investig. Dermatol. 1961, 36, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.; Slominski, A.; Pawelek, J.M.; Jimbow, K.; Gilchrest, B.A. What controls melanogenesis? Exp. Dermatol. 1998, 7, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B.; Breathnach, A.S. The epidermal melanin unit system. Dermatol. Wochenschr. 1963, 147, 481–489. [Google Scholar] [PubMed]
- Jimbow, K.; Quevedo, W.C.; Fitzpatrick, T.B.; Szabo, G. Some aspects of melanin biology: 1950–1975. J. Investig. Dermatol. 1976, 67, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Nakagawa, A.; Muramatsu, T.; Yamashina, Y.; Shirai, T.; Hashimoto, M.W.; Ishigaki, Y.; Ohnishi, T.; Mori, T. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J. Investig. Dermatol. 1998, 110, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Agar, N.; Young, A.R. Melanogenesis: A photoprotective response to DNA damage? Mutat. Res. 2005, 571, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Bush, W.D.; Simon, J.D. Quantification of Ca(2+) binding to melanin supports the hypothesis that melanosomes serve a functional role in regulating calcium homeostasis. Pigment Cell Res. 2007, 20, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Costin, G.-E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. Fed. Am. Soc. Exp. Biol. J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239. [Google Scholar] [CrossRef]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y.; Kawachi, Y.; Otsuka, F. Oral intake of proanthocyanidin-rich extract from grape seeds improves chloasma. Phytother. Res. 2004, 18, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Trouba, K.J.; Hamadeh, H.K.; Amin, R.P.; Germolec, D.R. Oxidative stress and its role in skin disease. Antioxid. Redox Signal. 2002, 4, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Jabbar, Z.; Athar, M.; Alam, M.S. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem. Toxicol. 2006, 44, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2016. [Google Scholar] [CrossRef] [PubMed]
- Prota, G. The role of peroxidase in melanogenesis revisited. Pigment Cell Res. 1992, 3, 25–31. [Google Scholar] [CrossRef]
- Baurin, N.; Arnoult, E.; Scior, T.; Do, Q.T.; Bernard, P. Preliminary screening of some tropical plants for anti-tyrosinase activity. J. Ethnopharmacol. 2002, 82, 155–158. [Google Scholar] [CrossRef]
- Jimbow, K.; Alena, F.; Dixon, W.; Hara, H. Regulatory factors of pheo- and eumelanogenesis in melanogenic compartments. Pigment Cell Res. 1992, 3, 36–42. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: A comparative review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Prota, G. Progress in the chemistry of melanins and related metabolites. Med. Res. Rev. 1988, 8, 525–556. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J. Determination of melanin synthetic pathways. J. Investig. Dermatol. 2011, 131, E8–E11. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Brenner, M.; Hearing, V.J. The regulation of skin pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. Soleil et peau. J. Médecine Esthét. 1975, 2, 33–34. (In French) [Google Scholar]
- Mapunya, M.B.; Nikolova, R.V.; Lall, N. Melanogenesis and antityrosinase activity of selected South African plants. Evid.-Based Complement. Altern. Med. 2012, 2012, e374017. [Google Scholar] [CrossRef] [PubMed]
- Almeda, F.; Astorga, L.; Orellana, A.; Sampuel, L.; Sierra, P.; Gaitán, I.; Cáceres, A. Piper genus: Source of natural products with anti-tyrosinase activity favored in phytocosmetics. Int. J. Phytocosmetics Nat. Ingred. 2015, 2, 6. [Google Scholar] [CrossRef]
- Krause, W. Drug-induced hyperpigmentation: A systematic review. J. Dtsch. Dermatol. Ges. 2013, 11, 644–651. [Google Scholar] [PubMed]
- Kim, S.S.; Kim, M.-J.; Choi, Y.H.; Kim, B.K.; Kim, K.S.; Park, K.J.; Park, S.M.; Lee, N.H.; Hyun, C.-G. Down-regulation of tyrosinase, TRP-1, TRP-2 and MITF expressions by citrus press-cakes in murine B16 F10 melanoma. Asian Pac. J. Trop. Biomed. 2013, 3, 617–622. [Google Scholar] [CrossRef]
- Chang, T.-S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Inhibitors of melanogenesis: A patent review (2009–2014). Expert Opin. Ther. Pat. 2015, 25, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Hershey, C.L.; Fisher, D.E. Mitf and Tfe3: Members of a b-HLH-ZIP transcription factor family essential for osteoclast development and function. Bone 2004, 34, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Park, K.C. Current clinical use of depigmenting agents. Dermatol. Sin. 2014, 32, 205–210. [Google Scholar] [CrossRef]
- Murase, D.; Hachiya, A.; Takano, K.; Hicks, R.; Visscher, M.O.; Kitahara, T.; Hase, T.; Takema, Y.; Yoshimori, T. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J. Investig. Dermatol. 2013, 133, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, S.H. Separation, purification, and properties of two tyrosinases from hamster melanoma. J. Biol. Chem. 1963, 238, 2351–2357. [Google Scholar] [PubMed]
- Curto, E.V.; Kwong, C.; Hermersdörfer, H.; Glatt, H.; Santis, C.; Virador, V.; Hearing, V.J.; Dooley, T.P. Inhibitors of mammalian melanocyte tyrosinase: In vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem. Pharmacol. 1999, 57, 663–672. [Google Scholar] [CrossRef]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem. 2003, 51, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.-S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, J.; Song, K.; Kim, H.G.; Koh, J.-S.; Boo, Y.C. Screening of plant extracts for human tyrosinase inhibiting effects. Int. J. Cosmet. Sci. 2012, 34, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.I.; Townsend, D.; Olds, D.P.; King, R.A. Dopachrome oxidoreductase: A new enzyme in the pigment pathway. J. Investig. Dermatol. 1984, 83, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Guyonneau, L.; Murisier, F.; Rossier, A.; Moulin, A.; Beermann, F. Melanocytes and pigmentation are affected in dopachrome tautomerase knockout mice. Mol. Cell. Biol. 2004, 24, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Lee, Y.S.; Hwang, S.; Kim, S.; Hwang, J.S.; Kim, T.-Y. N-(3,5-Dimethylphenyl)-3-Methoxybenzamide (A3B5) targets TRP-2 and inhibits melanogenesis and melanoma growth. J. Investig. Dermatol. 2011, 131, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Yoon, W.J.; Hyun, C.-G.; Lee, N.H. Down-regulation of tyrosinase, TRP-2 and MITF expressions by Neolitsea aciculata extract in murine B16 F10 melanoma. Int. J. Pharmacol. 2010, 6, 290–295. [Google Scholar] [CrossRef]
- Aroca, P.; Solano, F.; García-Borrón, J.C.; Lozano, J.A. A new spectrophotometric assay for dopachrome tautomerase. J. Biochem. Biophys. Methods 1990, 21, 35–46. [Google Scholar] [CrossRef]
- Takahashi, M.; Takara, K.; Toyozato, T.; Wada, K. A novel bioactive chalcone of Morus australis inhibits tyrosinase activity and melanin biosynthesis in B16 melanoma cells. J. Oleo Sci. 2012, 61, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-T.; Chang, W.-L.; Chang, C.-T.; Hsu, S.-L.; Lin, Y.-C.; Shih, Y. Cinnamomum cassia essential oil inhibits α-MSH-induced melanin production and oxidative stress in murine B16 melanoma cells. Int. J. Mol. Sci. 2013, 14, 19186–19201. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.T.S.; Arroteia, K.F.; Santos, I.A.; Andres, E.; Medina, S.P.H.; Ferrari, C.R.; Lourenço, C.B.; Biaggio, R.M.T.T.; Moreira, P.L. Schinus terebinthifolius Raddi extract and linoleic acid from Passiflora edulis synergistically decrease melanin synthesis in B16 cells and reconstituted epidermis. Int. J. Cosmet. Sci. 2012, 34, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Hegde, B.; Vadnal, P.; Sanghavi, J.; Korde, V.; Kulkarni-Almeida, A.A.; Dagia, N.M. Vitamin E is a MIF inhibitor. Biochem. Biophys. Res. Commun. 2012, 418, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Nicolaÿ, J.-F.; Levrat, B. A keratinocytes-melanocytes coculture system for the evaluation of active ingredients’ effects on UV-induced melanogenesis. Int. J. Cosmet. Sci. 2003, 25, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Smit, N.P.M.; Kolb, A.M.; Régnier, M.; Pavel, S.; Schmidt, R. Keratinocytes control the pheo/eumelanin ratio in cultured normal human melanocytes. Pigment Cell Res. 2002, 15, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Lehraiki, A.; Abbe, P.; Cerezo, M.; Rouaud, F.; Regazzetti, C.; Chignon-Sicard, B.; Passeron, T.; Bertolotto, C.; Ballotti, R.; Rocchi, S. Inhibition of melanogenesis by the antidiabetic metformin. J. Investig. Dermatol. 2014, 134, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, I.; Angers, L.; Jean, J.; Pouliot, R. Pigmented skin models: Understand the mechanisms of melanocytes. In Regenerative Medicine and Tissue Engineering; Andrades, J.A., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Lin, V.C.; Ding, H.-Y.; Tsai, P.-C.; Wu, J.-Y.; Lu, Y.-H.; Chang, T.-S. In vitro and in vivo melanogenesis inhibition by biochanin A from Trifolium pratense. Biosci. Biotechnol. Biochem. 2011, 75, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-H.; Ko, S.-C.; Kim, D.; Jeon, Y.-J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol. 2011, 38, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Kim, J.-H.; Ko, D.H.; Kim, C.-H.; Hwang, J.-S.; Ahn, S.; Kim, S.Y.; Kim, C.-D.; Lee, J.-H.; Yoon, T.-J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Seo, J.O.; Do, M.H.; Ji, E.; Baek, S.-H.; Kim, S.Y. Resveratrol-enriched rice down-regulates melanin synthesis in UVB-induced guinea pigs epidermal skin tissue. Biomol. Ther. 2014, 22, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Ikeda, K.; Saito, M. Inhibitory effect of rose hip (Rosa canina L.) on melanogenesis in mouse melanoma cells and on pigmentation in brown guinea pigs. Biosci. Biotechnol. Biochem. 2011, 75, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Nair, X.; Tramposch, K. The Yucatan miniature swine as an in vivo model for screening skin depigmentation. J. Dermatol. Sci. 1991, 2, 428–433. [Google Scholar] [CrossRef]
- Nair, X.; Tramposch, K. Effect of single UVR exposure on skin pigmentation and melanocyte morphology in the Yucatan miniature swine. J. Investig. Dermatol. 1990, 94, 558. [Google Scholar]
- Peal, D.S.; Peterson, R.T.; Milan, D. Small molecule screening in zebrafish. J. Cardiovasc. Transl. Res. 2010, 3, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.V.; Ding, H.-Y.; Kuo, S.-Y.; Chin, L.-W.; Wu, J.-Y.; Chang, T.-S. Evaluation of in vitro and in vivo depigmenting activity of raspberry ketone from Rheum officinale. Int. J. Mol. Sci. 2011, 12, 4819–4835. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Kawai, M.; Mishima, Y.; Motegi, I. Differential analysis of experimental hypermelanosis induced by UVB, PUVA, and allergic contact dermatitis using a brownish guinea pig model. Arch. Dermatol. Res. 1986, 278, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Schwarz, R.; Neurand, K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr. Probl. Dermatol. 1978, 7, 39–52. [Google Scholar] [PubMed]
- Montagna, W.; Yun, J.S. The skin of the domestic pig. J. Investig. Dermatol. 1964, 42, 11–21. [Google Scholar] [CrossRef] [PubMed]
- David, L.T. Histology of the skin of the Mexican hairless swine (Sus scrofa). Am. J. Anat. 1932, 50, 283–292. [Google Scholar] [CrossRef]
- Abella, M.L.; de Rigal, J.; Neveux, S. A simple experimental method to study depigmenting agents. Int. J. Cosmet. Sci. 2007, 29, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Benz, M.; Mayr, A.; Gefeller, O.; Pfahlberg, A. Assessing skin pigmentation in epidemiological studies: The reliability of measurements under different conditions. Skin Res. Technol. 2013, 19, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Hoshino, T.; Chen, C.J.; E, Y.; Yabe, S.; Liu, W. The evaluation of whitening efficacy of cosmetic products using a human skin pigmentation spot model. Skin Res. Technol. 2009, 15, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Hashizume, E.; Chan, G.P.; Kamimura, A. Skin-whitening and skin-condition-improving effects of topical oxidized glutathione: A double-blind and placebo-controlled clinical trial in healthy women. Clin. Cosmet. Investig. Dermatol. 2014, 7, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Sato, K.; Aiba-Kojima, E.; Matsumoto, D.; Machino, C.; Nagase, T.; Gonda, K.; Koshima, I. Repeated treatment protocols for melasma and acquired dermal melanocytosis. Dermatol. Surg. 2006, 32, 365–371. [Google Scholar] [PubMed]
- Orlow, S.J.; Chakraborty, A.K.; Pawelek, J.M. Retinoic acid is a potent inhibitor of inducible pigmentation in murine and hamster melanoma cell lines. J. Investig. Dermatol. 1990, 94, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, J.-P. Retinoid therapy of pigmentary disorders. Dermatol. Ther. 2006, 19, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Weldon, M.M.; Smolinski, M.S.; Maroufi, A.; Hasty, B.W.; Gilliss, D.L.; Boulanger, L.L.; Balluz, L.S.; Dutton, R.J. Mercury poisoning associated with a Mexican beauty cream. West. J. Med. 2000, 173, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Pop, C.; Vlase, L.; Tamas, M. Natural resources containing arbutin. Determination of arbutin in the leaves of Bergenia crassifolia (L.) Fritsch. acclimated in Romania. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 129–132. [Google Scholar]
- Qin, L.; Wu, Y.; Liu, Y.; Chen, Y.; Zhang, P. Dual effects of alpha-arbutin on monophenolase and diphenolase activities of mushroom tyrosinase. PLoS ONE 2014, 9, e109398. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, J.; Chazarra, S.; Garcia-Carmona, F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J. Pharm. Pharmacol. 1994, 46, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. From miso, saké and shoyu to cosmetics: A century of science for kojic acid. Nat. Prod. Rep. 2006, 23, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.M.; Chen, H.W.; Huang, Y.H.; Chan, S.Y.; Chen, C.C.; Wu, W.C.; Wen, K.C. Melanogenesis and natural hypopigmentation agents. In Melanin: Biosynthesis, functions and health effects; eBook; Ma, X.-P., Sun, X.-X., Eds.; NOVA Science Publisher: Hauppauge, NY, USA, 2012; pp. 1–76. [Google Scholar]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Arjinpathana, N.; Asawanonda, P. Glutathione as an oral whitening agent: A randomized, double-blind, placebo-controlled study. J. Dermatol. Treat. 2012, 23, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Sahashi, Y.; Aritro, M.; Hasegawa, S.; Akimoto, K.; Ninomiya, S.; Sakaguchi, Y.; Seyama, Y. Effect of simultaneous administration of vitamin C, L-cysteine and vitamin E on the melanogenesis. BioFactors 2004, 21, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Sakai, C.; Kondoh, S.; Yonemoto, K.; Nishiyama, S.; Tagawa, M.; Murata, T.; Ohnuma, T.; Quigley, J.; Dorsky, A.; et al. Inhibitory effect of magnesium l-ascorbyl-2-phosphate (VC-PMG) on melanogenesis in vitro and in vivo. J. Am. Acad. Dermatol. 1996, 34, 29–33. [Google Scholar] [CrossRef]
- Régnier, M.; Tremblaye, C.; Schmidt, R. Vitamin C affects melanocyte dendricity via keratinocytes. Pigment Cell Res. 2005, 18, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.G.; Bissett, D.L.; Boissy, R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Solís, J.; Castanedo-Cázares, J.P.; Torres-Álvarez, B.; Oros-Ovalle, C.; Fuentes-Ahumada, C.; González, F.J.; Martínez-Ramírez, J.D.; Moncada, B. A double-blind, randomized clinical trial of niacinamide 4% versus hydroquinone 4% in the treatment of melasma. Dermatol. Res. Pract. 2011, 2011, e379173. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. A theory for the mechanism of action of the alpha-hydroxy acids applied to the skin. Med. Hypotheses 1999, 53, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Usuki, A.; Ohashi, A.; Sato, H.; Ochiai, Y.; Ichihashi, M.; Funasaka, Y. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp. Dermatol. 2003, 12 (Suppl. S2), 43–50. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.-S.; Cho, C.; Hong, M.-C.; Shin, M.-K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Charlín, R.; Barcaui, C.B.; Kac, B.K.; Soares, D.B.; Rabello-Fonseca, R.; Azulay-Abulafia, L. Hydroquinone-induced exogenous ochronosis: A report of four cases and usefulness of dermoscopy. Int. J. Dermatol. 2008, 47, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Olumide, Y.M.; Akinkugbe, A.O.; Altraide, D.; Mohammed, T.; Ahamefule, N.; Ayanlowo, S.; Onyekonwu, C.; Essen, N. Complications of chronic use of skin lightening cosmetics. Int. J. Dermatol. 2008, 47, 344–353. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, J.L. Hydroquinone and its analogues in dermatology—A risk-benefit viewpoint. J. Cosmet. Dermatol. 2006, 5, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Dadzie, O.E.; Petit, A. Skin bleaching: Highlighting the misuse of cutaneous depigmenting agents. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 741–750. [Google Scholar] [CrossRef] [PubMed]
- AFSSAPS. Évaluation des Risques Liés à la Dépigmentation Volontaire. Available online: http://ansm.sante.fr/content/download/36916/483991/version/1/file/Rapport-depigmentation2011.pdf (accessed on 21 September 2016).
- Liste des Produits Eclaircissants de la Peau Non Conformes et Dangereux Identifiés en France, Contenant de L’hydroquinone. Available online: http://ansm.sante.fr/content/download/36918/484005/version/1/file/Liste-produits+-depigmentatio+-Afssaps-DGCCRF.pdf (accessed on 21 September 2016).
- ANSM. Suspension de la Mise sur le Marché des Produits Eclaircissants de la Peau Présentés en Solution Injectable—Point D’information. Available online: http://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Suspension-de-la-mise-sur-le-marche-des-produits-eclaircissants-de-la-peau-presentes-en-solution-injectable-Point-d-Information (accessed on 21 September 2016).
- FDA. Consumer Health Information—Injectable Skin Lightening Products: What You Should Know. Available online: http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/UCM460999.pdf (accessed on 21 September 2016).
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF (accessed on 21 September 2016).
- ANSM. Groupe de Travail Reproduction, Grossesse et Allaitement GT252015043. Available online: http://ansm.sante.fr/var/ansm_site/storage/original/application/a713e233daf32e61474bf1462500a0b1.pdf (accessed on 21 September 2016).
- Blaut, M.; Braune, A.; Wunderlich, S.; Sauer, P.; Schneider, H.; Glatt, H. Mutagenicity of arbutin in mammalian cells after activation by human intestinal bacteria. Food Chem. Toxicol. 2006, 44, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- SCCS/1481/12—Opinion on Kojic Acid. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_mi_015.pdf (accessed on 21 September 2016).
- Bains, V.K.; Loomba, K.; Loomba, A.; Bains, R. Mercury sensitisation: Review, relevance and a clinical report. Br. Dent. J. 2008, 205, 373–378. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Mercury in Skin Lightening Products. Available online: http://www.who.int/ipcs/assessment/public_health/mercury_flyer.pdf (accessed on 21 September 2016).
- Engler, D.E. Mercury “bleaching” creams. J. Am. Acad. Dermatol. 2005, 52, 1113–1114. [Google Scholar] [CrossRef] [PubMed]
- Tlacuilo-Parra, A.; Guevara-Gutiérrez, E.; Luna-Encinas, J.A. Percutaneous mercury poisoning with a beauty cream in Mexico. J. Am. Acad. Dermatol. 2001, 45, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Counter, S.A.; Buchanan, L.H. Mercury exposure in children: A review. Toxicol. Appl. Pharmacol. 2004, 198, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Mahé, A.; Ly, F.; Perret, J.-L. Systemic complications of the cosmetic use of skin-bleaching products. Int. J. Dermatol. 2005, 44 (Suppl. S1), 37–38. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, B.; Van Hoeck, E.; Rogiers, V.; Courselle, P.; De Beer, J.O.; De Paepe, K.; Deconinck, E. Characterization of suspected illegal skin whitening cosmetics. J. Pharm. Biomed. Anal. 2014, 90, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Kawai, K.; Kawai, K. Contact allergy to kojic acid in skin care products. Contact Dermat. 1995, 32, 9–13. [Google Scholar] [CrossRef]
- DFI. Ordonnance du DFI sur les Cosmétiques RS 817.023.31. Available online: https://www.admin.ch/opc/fr/classified-compilation/20050180/201510010000/817.023.31.pdf (accessed on 21 September 2016).
- Pauwels, M.; Rogiers, V. Human health safety evaluation of cosmetics in the EU: A legally imposed challenge to science. Toxicol. Appl. Pharmacol. 2010, 243, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Batubara, I.; Darusman, L.K.; Mitsunaga, T.; Rahminiwati, M.; Djauhari, E. Potency of Indonesian medicinal plants as tyrosinase inhibitor and antioxidant agent. J. Biol. Sci. 2010, 10, 138–144. [Google Scholar] [CrossRef]
- Nugroho, A.; Choi, J.-K.; Park, J.-H.; Lee, K.-T.; Cha, B.C.; Park, H.-J. Two new flavonol glycosides from Lamium amplexicaule L. and their in vitro free radical scavenging and tyrosinase inhibitory activities. Planta Med. 2009, 75, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Nishio, H.; Kubota, Y.; Mizoguchi, M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res. 1998, 11, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-P.; Chen, Q.-X.; Huang, H.; Wang, H.-Z.; Zhang, R.-Q. Inhibitory effects of some flavonoids on the activity of mushroom tyrosinase. Biochem. Mosc. 2003, 68, 487–491. [Google Scholar] [CrossRef]
- Jones, K.; Hughes, J.; Hong, M.; Jia, Q.; Orndorff, S. Modulation of melanogenesis by aloesin: A competitive inhibitor of tyrosinase. Pigment Cell Res. 2002, 15, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, S.-K.; Kim, J.-E.; Chung, M.-H.; Park, Y.-I. Aloesin inhibits hyperpigmentation induced by UV radiation. Clin. Exp. Dermatol. 2002, 27, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Leu, Y.-L.; Hwang, T.-L.; Hu, J.-W.; Fang, J.-Y. Anthraquinones from Polygonum cuspidatum as tyrosinase inhibitors for dermal use. Phytother. Res. 2008, 22, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Devkota, K.P.; Khan, M.T.H.; Ranjit, R.; Lannang, A.M.; Samreen; Choudhary, M.I. Tyrosinase inhibitory and antileishmanial constituents from the rhizomes of Paris polyphylla. Nat. Prod. Res. 2007, 21, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.-C.; Wu, C.-H.; Yen, G.-C. Bioactivity and potential health benefits of licorice. J. Agric. Food Chem. 2014, 62, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-H.; Chou, T.-H.; Ding, H.-Y. Inhibition of melanogenesis by a novel origanoside from Origanum vulgare. J. Dermatol. Sci. 2010, 57, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.H.; Ryu, S.Y.; Choi, E.J.; Kang, S.H.; Chang, I.M.; Min, K.R.; Kim, Y. Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 1998, 243, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Ryu, Y.B.; Curtis-Long, M.J.; Ryu, H.W.; Baek, Y.S.; Kang, J.E.; Lee, W.S.; Park, K.H. Tyrosinase inhibitory polyphenols from roots of Morus lhou. J. Agric. Food Chem. 2009, 57, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, S.Y.; Kim, H.; Hwang, J.S.; Lee, B.G.; Gao, J.J.; Kim, S.Y. Mulberroside F isolated from the leaves of Morus alba inhibits melanin biosynthesis. Biol. Pharm. Bull. 2002, 25, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.B.; Lee, D.Y.; Kim, T.B.; Kim, S.H.; Kim, H.J.; Kim, J.; Sung, S.H. Prediction of tyrosinase inhibitory activities of Morus alba root bark extracts from HPLC fingerprints. Microchem. J. 2013, 110, 731–738. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.Y.; Hong, C.Y.; Gwak, K.S.; Park, M.J.; Smith, D.; Choi, I.G. Whitening and antioxidant activities of bornyl acetate and nezukol fractionated from Cryptomeria japonica essential oil. Int. J. Cosmet. Sci. 2013, 35, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, Y.; Tao, L.; Tao, X.; Su, X.; Wei, D. Tyrosinase inhibitory effects and inhibition mechanisms of nobiletin and hesperidin from citrus peel crude extracts. J. Enzyme Inhib. Med. Chem. 2007, 22, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Kinst-Hori, I. Tyrosinase inhibitors from anise oil. J. Agric. Food Chem. 1998, 46, 1268–1271. [Google Scholar] [CrossRef]
- Lee, H.-S. Tyrosinase inhibitors of Pulsatilla cernua root-derived materials. J. Agric. Food Chem. 2002, 50, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.H.; Khan, S.B.; Ather, A. Tyrosinase inhibitory cycloartane type triterpenoids from the methanol extract of the whole plant of Amberboa ramosa Jafri and their structure-activity relationship. Bioorg. Med. Chem. 2006, 14, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Hussain, H.; Hussain, J.; Bukhari, I.A.; Khan, M.T.H.; Choudhary, M.I.; Gilani, A.H.; Ahmad, V.U. Tyrosinase inhibitory pentacyclic triterpenes and analgesic and spasmolytic activities of methanol extracts of Rhododendron collettianum. Phytother. Res. 2007, 21, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Paine, C.; Sharlow, E.; Liebel, F.; Eisinger, M.; Shapiro, S.; Seiberg, M. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J. Investig. Dermatol. 2001, 116, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Saeki, Y.; Kanamaru, A.; Matsumoto, K.; Tada, A. The inhibitory effects of centaureidin on the outgrowth of dendrites, melanosome transfer and melanogenesis in normal human melanocyte. Pigment Cell Res. 2003, 16, 593–593. [Google Scholar] [CrossRef]
- Ito, Y.; Kanamaru, A.; Tada, A. Effects of methylophiopogonanone B on melanosome transfer and dendrite retraction. J. Dermatol. Sci. 2006, 42, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, M.K.; Lee, U.; Kim, S.-K.; Kang, J.S.; Choi, H.D.; Son, B.W. Myrothenones A and B, cyclopentenone derivatives with tyrosinase inhibitory activity from the marine-derived fungus Myrothecium sp. Chem. Pharm. Bull. (Tokyo) 2005, 53, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, D.; Arciuli, M.; Arena, M.P.; Benvenuti, S.; Gallone, A. Chemical composition and the anti-melanogenic potential of different essential oils. Flavour Fragr. J. 2016, 31, 255–261. [Google Scholar] [CrossRef]
- Del Garcia-Molina, M.; Muñoz-Muñoz, J.L.; Garcia-Molina, F.; García-Ruiz, P.A.; Garcia-Canovas, F. Action of tyrosinase on ortho-substituted phenols: Possible influence on browning and melanogenesis. J. Agric. Food Chem. 2012, 60, 6447–6453. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-Y.; Lin, C.-C.; Wang, H.-Y.; Shih, Y.; Chou, S.-T. The melanogenesis alteration effects of Achillea millefolium L. essential oil and linalyl acetate: Involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS ONE 2014, 9, e95186. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Kubo, I. Effects of thymol on mushroom tyrosinase-catalyzed melanin formation. J. Agric. Food Chem. 2011, 59, 8908–8914. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ruiz, C.V.; del Garcia-Molina, M.; Serrano, J.T.; Tomas-Martinez, V.; Garcia-Canovas, F. Discrimination between alternative substrates and inhibitors of tyrosinase. J. Agric. Food Chem. 2015, 63, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Huang, Y.-C.; Tsai, M.-L.; Cheng, C.-Y.; Liu, L.-L.; Yen, Y.-W.; Chen, W.-L. Inhibition of melanogenesis by β-caryophyllene from lime mint essential oil in mouse B16 melanoma cells. Int. J. Cosmet. Sci. 2015, 37, 550–554. [Google Scholar] [CrossRef] [PubMed]
- D’enfert, V. The Nagoya Protocol imposes new rules. Expr. Cosmét. 2013, 24, 40–42. [Google Scholar]
- Hwang, T.-L.; Chen, H.-Y.; Changchien, T.-T.; Wang, C.-C.; Wu, C.-M. The cytotoxicity of mercury chloride to the keratinocytes is associated with metallothionein expression. Biomed. Rep. 2013, 1, 379–382. [Google Scholar] [PubMed]
- Inoue, Y.; Hasegawa, S.; Yamada, T.; Date, Y.; Mizutani, H.; Nakata, S.; Matsunaga, K.; Akamatsu, H. Analysis of the effects of hydroquinone and arbutin on the differentiation of melanocytes. Biol. Pharm. Bull. 2013, 36, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-L.; Huang Liu, R.; Sheu, J.-N.; Chen, S.-T.; Sinchaikul, S.; Tsay, G.J. Toxicogenomics of kojic acid on gene expression profiling of a375 human malignant melanoma cells. Biol. Pharm. Bull. 2006, 29, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Osborne, R.; Perkins, M.A. In vitro skin irritation testing with human skin cell cultures. Toxicol. In Vitro 1991, 5, 563–567. [Google Scholar] [CrossRef]
- Chen, W.-C.; Tseng, T.-S.; Hsiao, N.-W.; Lin, Y.-L.; Wen, Z.-H.; Tsai, C.-C.; Lee, Y.-C.; Lin, H.-H.; Tsai, K.-C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef] [PubMed]
- Secrétariat de la Convention sur la Diversité Biologique. Protocole de Nagoya sur L’accès aux Ressources Génétiques et le Partage Juste et Equitable des Avantages Découlant de Leur Utilisation Relatif à la Convention sur la Diversité Biologique: Texte et Annexe. ISBN: 92-9225-307-7. Available online: http://www.ecolex.org/server2.php/libcat/docs/LI/MON-086966FR.pdf (accessed on 22 September 2016).
- Lee, K.T.; Kim, B.J.; Kim, J.H.; Heo, M.Y.; Kim, H.P. Biological screening of 100 plant extracts for cosmetic use (I): Inhibitory activities of tyrosinase and DOPA auto-oxidation. Int. J. Cosmet. Sci. 1997, 19, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.A.; Duperret, E.K.; Zhang, J.; Sadeghi, R.; Dahal, A.; O’Brien, K.T.; Cookson, R.; Winkler, J.D.; Ridky, T.W. Sex steroids regulate skin pigmentation through nonclassical membrane-bound receptors. eLife 2016, 5, e15104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Yang, K.C. Comparison of the frequency-doubled Q-switched Nd:YAG laser and 35% trichloroacetic acid for the treatment of face lentigines. Dermatol. Surg. 1999, 25, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Stem, R.S.; Dover, J.S.; Levin, J.A.; Arndt, K.A. Laser therapy versus cryotherapy of lentigines: A comparative trial. J. Am. Acad. Dermatol. 1994, 30, 985–987. [Google Scholar] [CrossRef]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Dover, J.S.; Arndt, K.A. Laser treatment of pigmented lesions: How far have we gone? Arch. Dermatol. 2000, 136, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Kunachak, S.; Leelaudomlipi, P.; Wongwaisayawan, S. Dermabrasion: A curative treatment for melasma. Aesthet. Plast. Surg. 2001, 25, 114–117. [Google Scholar] [CrossRef]

| Substance | Chemical Structures (Examples) | Mechanisms of Action & Usages | References |
|---|---|---|---|
| Retinoids |  tretinoin | - interfere with melanosomes transfer; - increase keratinocytes turnover; - inhibit tyrosinase transcription; - used to treat melasma | [2,111,112,113] |
 isoretinoin | |||
| Hydroquinone and ethers derivatives |  hydroquinone | - used to treat hypermelanoses; - used alone or in combination with tretinoin to prevent sun- or hormone-induced melasma; - authorized in cosmetics until 2001 | [19,23] |
 monobenzyl ether of hydroquinone | |||
| Mercury salts |  mercury (II) chloride | - compete with copper in the tyrosinase’s active site; - inhibit the production of l-dopaquinone | [114] |
 ammoniated mercury | |||
| Arbutin (hydroquinone-β-d-glucoside) |  | - blocks the monophenolase activity; - decreases melanin content with little cytotoxicity evidence; - occurs naturally in Morus, Arctostaphylos, Vaccinus, Pyrus and Lathyrus species; - exists in two isomers: the α- one, offering higher stability over the β- one, is the preferred form for skin lightening | [115,116] |
| Kojic acid |  | - chelates copper ions, essential cofactors for tyrosinase activity; - inhibits the polymerization of DHI and DHICA; - highly unstable upon exposure to air or sunlight; - usually replaced in cosmetic formulations by its dipalmitate stable derivative | [117,118,119] |
| Azelaic acid |  | - inhibits the tyrosinase activity; - displays cytotoxic effect on human melanocytes | [2,23,120] |
| Glutathione |  | - sometimes combined with other agents like vitamin C to increase its absorption, or with antioxidants like vitamin E | [121] |
| Vitamins |  vitamin C | - accelerate epidermal turnover; - ascorbic acid notably reduces l-dopaquinone back to DOPA; - ascorbic acid interacts with copper in the tyrosinase’s active site; - highly unstable in aqueous medias, ascorbic acid is usually encapsulated or replaced in cosmetic formulations by derivatives such as MgAP; - magnesium ascorbyl phosphate (MgAP) affects, in a reversible manner, the melanocytes morphology, which lose their dendritic structure, hence impairing the melanocytes-keratinocytes contacts and reducing the melanin transfer; - niacinamide interferes with melanosomes transfer from melanocytes to keratinocytes and possesses antioxidant activity | [2,19,122,123,124,125,126] |
 vitamin E | |||
 niacinamide = vitamin B3 | |||
| Alpha hydroxy acids (AHAs or fruit acids) |  glycolic acid | - accelerate epidermal turnover; - remove unhealthy or abnormal layers of superficial skin cells (desquamation); - facilitate the dermal penetration of other whitening agents | [127,128,129] |
 lactic acid | |||
| Corticosteroids |  clobetasol propionate | - modulate the activation of MC1R | [32] |
 betamethasone 17-valerate |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin Whitening Cosmetics: Feedback and Challenges in the Development of Natural Skin Lighteners. Cosmetics 2016, 3, 36. https://doi.org/10.3390/cosmetics3040036
Burger P, Landreau A, Azoulay S, Michel T, Fernandez X. Skin Whitening Cosmetics: Feedback and Challenges in the Development of Natural Skin Lighteners. Cosmetics. 2016; 3(4):36. https://doi.org/10.3390/cosmetics3040036
Chicago/Turabian StyleBurger, Pauline, Anne Landreau, Stéphane Azoulay, Thomas Michel, and Xavier Fernandez. 2016. "Skin Whitening Cosmetics: Feedback and Challenges in the Development of Natural Skin Lighteners" Cosmetics 3, no. 4: 36. https://doi.org/10.3390/cosmetics3040036







