All-Solid-State Textile Batteries Made from Nano-Emulsion Conducting Polymer Inks for Wearable Electronics
Abstract
:1. Introduction
2. Results and Discussion
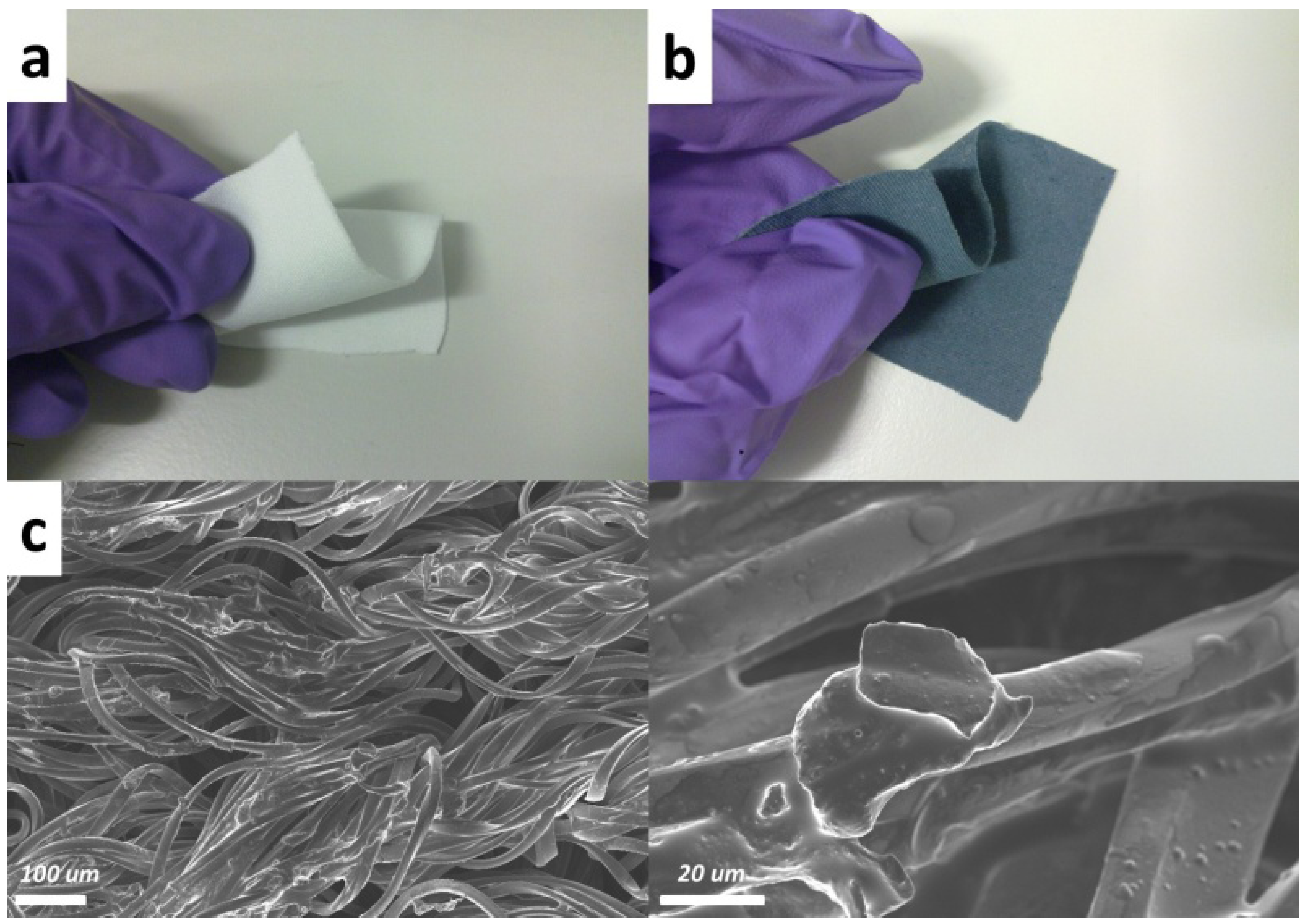
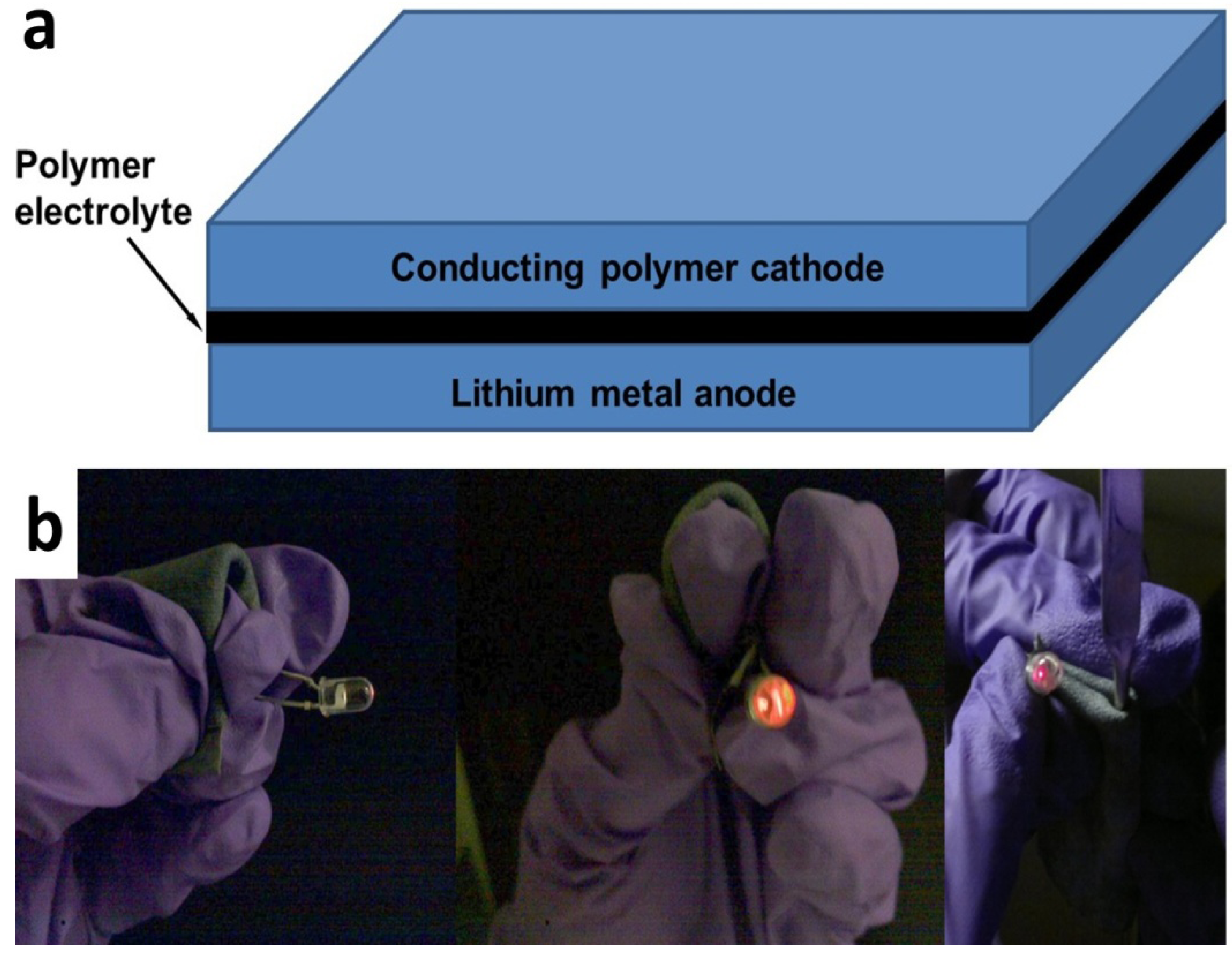
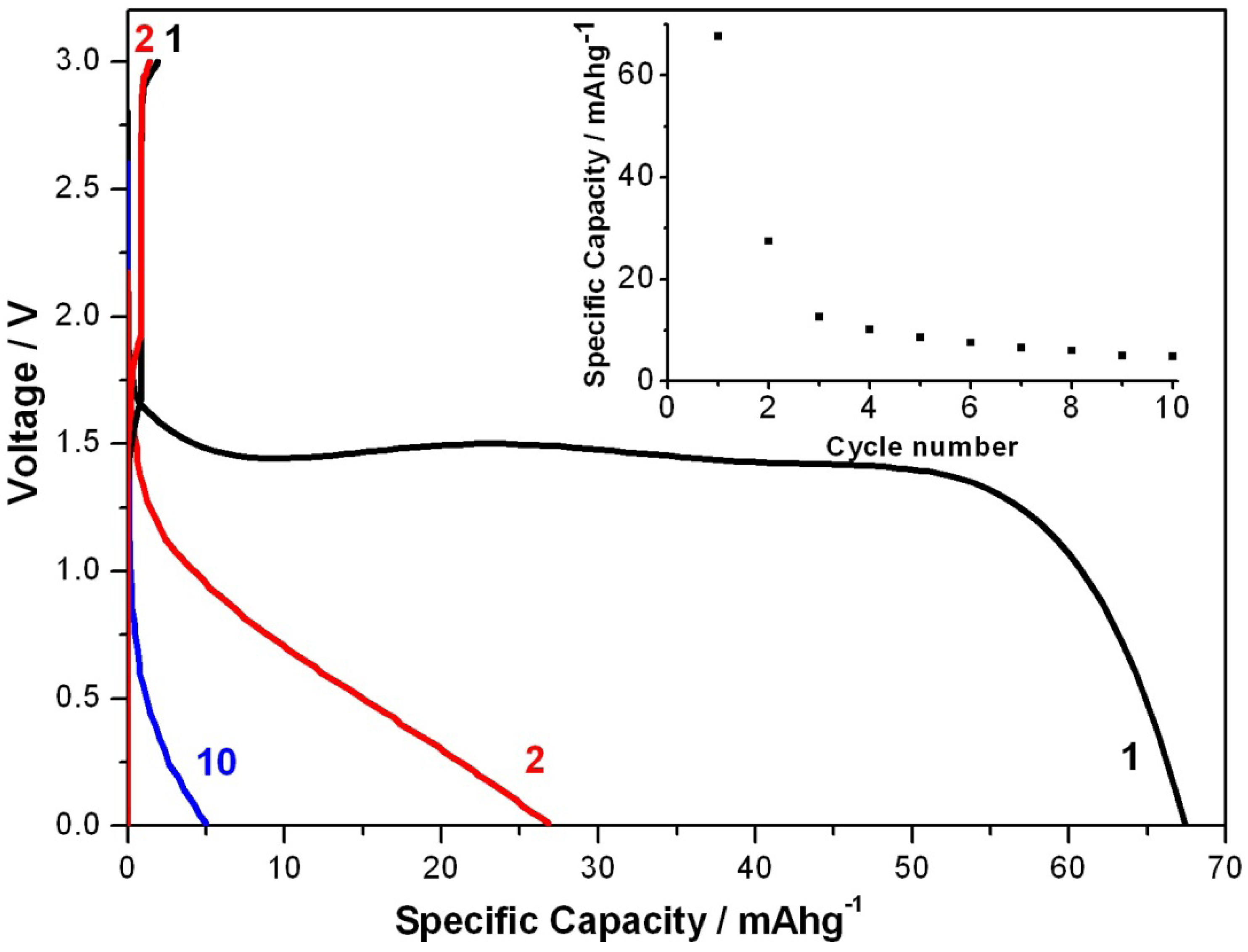
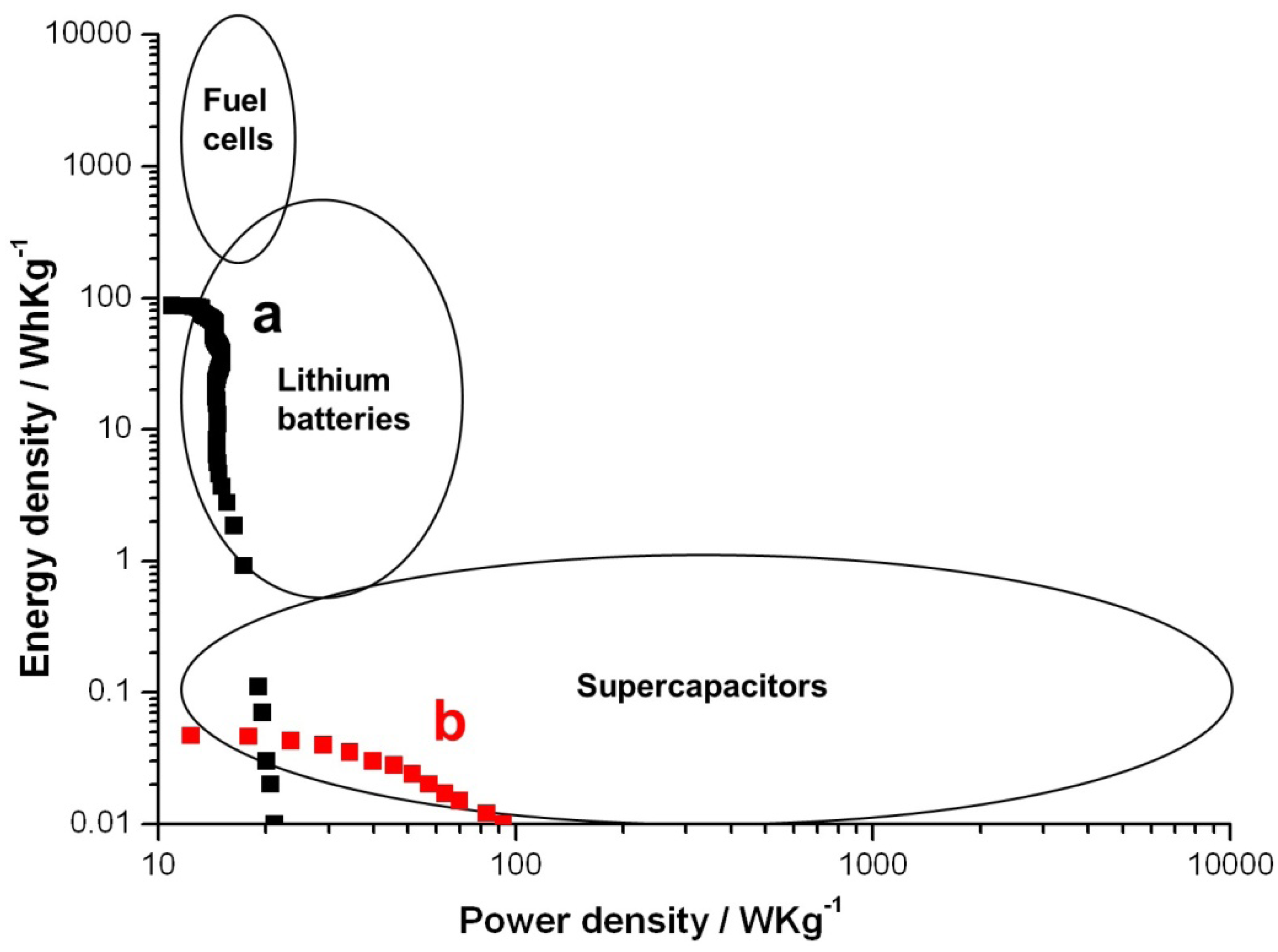
3. Experimental Section
4. Conclusions
References
- Shim, B.S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N.A. Smart electronic yarns and wearable fabrics for human biomonitoring made by carbon nanotube coating with polyelectrolytes. Nano Lett. 2008, 8, 4151–4157. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Xie, X.; Liu, N.; Yang, Y.; Wu, H.; Yao, Y.; Pasta, M.; Alshareef, H.N.; Cui, Y. Symmetrical MnO2-carbon nanotube-textile nanostructures for wearable pseudocapacitors with high mass loading. ACS Nano 2011, 5, 8904–8913. [Google Scholar] [CrossRef]
- Yu, G.; Hu, L.; Vosgueritchian, M.; Wang, H.; Xie, X.; McDonough, J.R.; Cui, X.; Cui, Y.; Bao, Z. Solution-processed graphene/MnO2 nanostructured textiles for high-performance electrochemical capacitors. Nano Lett. 2011, 11, 2905–2911. [Google Scholar] [CrossRef]
- Hu, L.; Pasta, M.; Mantia, F.L.; Cui, L.; Jeong, S.; Deshazer, H.D.; Choi, J.W.; Han, S.M.; Cui, Y. Stretchable, porous, and conductive energy textiles. Nano Lett. 2010, 10, 708–714. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Nyholm, L.; Nyström, G.; Mihranyan, A.; Strømme, M. Toward flexible polymer and paper-based energy storage device. Adv. Mater. 2011, 23, 3751–3769. [Google Scholar]
- Razaq, A.; Nyholm, L.; Sjödin, M.; Strømme, M.; Mihranyan, A. Paper-based energy-storage devices comprising carbon fiber-reinforced polypyrrole-cladophora nanocellulose composite electrodes. Adv. Energy Mater. 2012, 4, 445–454. [Google Scholar]
- Novak, P.; Muller, K.; Santhanam, K.S.V.; Haas, O. Conducting polymers for battery applications. Chem. Rev. 1997, 97, 207–281. [Google Scholar]
- Shirakawa, H.; Lious, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar]
- MacDiarmid, A.G. Synthetic metals: A novel role for organic polymers (Nobel lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Li, C.; Peng, X.; Zhang, B.; Wang, B. An all-solid-state lithium/polyaniline rechargeable cell. J. Power Sources 1992, 39, 255–258. [Google Scholar] [CrossRef]
- Novak, P.; Inganas, O.; Bjorklund, R. Cycling behaviour of the polypyrrole-polyethylene oxide composite electrode. J. Power Sources 1987, 21, 17–24. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, K.; Mielczarek, K.; Hu, W.; Zakhidov, A. Nanoimprint of dehydrated PEDOT:PSS for organic photovoltaics. Nanotechnology 2011, 22, 485301. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, W.; Yue, Z.; Too, C.O.; Wallace, G.G. Buckled, stretchable polypyrrole electrodes for battery applications. Adv. Mater. 2011, 23, 3580–3584. [Google Scholar] [CrossRef]
- Wei, D.; Andrew, P.; Yang, H.; Jiang, J.; Ruan, W.; Han, D.; Niu, L.; Bower, C.; Ryhanen, T.; Rouvala, M.; Amaratunga, G.A.J.; Ivaska, A. Flexible solid state lithium batteries based on graphene inks. J. Mater. Chem. 2011, 21, 9762–9767. [Google Scholar]
- Kil, E.H.; Ha, H.J.; Lee, S.Y. A facile approach to fabricate self-standing gel-polymer electrolytes for flexible lithium-ion batteries by exploitation of UV-cured trivalent/monovalent acrylate polymer matrices. Macromol. Chem. Phys. 2011, 212, 2217–2223. [Google Scholar] [CrossRef]
- Nystrom, G.; Razaq, A.; Stromme, M.; Nyholm, L.; Mihranyan, A. Ultrafast all-polymer paper-based batteries. Nano Lett. 2009, 9, 3635–3639. [Google Scholar]
- Arbizzani, C.; Mastragostino, M. Polybithiophene as positive electrode in solid-state polyethylene oxide—LiClO4 lithium rechargeable battery. Electrochim. Acta 1990, 35, 251–254. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wei, D.; Cotton, D.; Ryhänen, T. All-Solid-State Textile Batteries Made from Nano-Emulsion Conducting Polymer Inks for Wearable Electronics. Nanomaterials 2012, 2, 268-274. https://doi.org/10.3390/nano2030268
Wei D, Cotton D, Ryhänen T. All-Solid-State Textile Batteries Made from Nano-Emulsion Conducting Polymer Inks for Wearable Electronics. Nanomaterials. 2012; 2(3):268-274. https://doi.org/10.3390/nano2030268
Chicago/Turabian StyleWei, Di, Darryl Cotton, and Tapani Ryhänen. 2012. "All-Solid-State Textile Batteries Made from Nano-Emulsion Conducting Polymer Inks for Wearable Electronics" Nanomaterials 2, no. 3: 268-274. https://doi.org/10.3390/nano2030268
APA StyleWei, D., Cotton, D., & Ryhänen, T. (2012). All-Solid-State Textile Batteries Made from Nano-Emulsion Conducting Polymer Inks for Wearable Electronics. Nanomaterials, 2(3), 268-274. https://doi.org/10.3390/nano2030268



