Tissue Engineering of Corneal Endothelium
Abstract
:1. Introduction
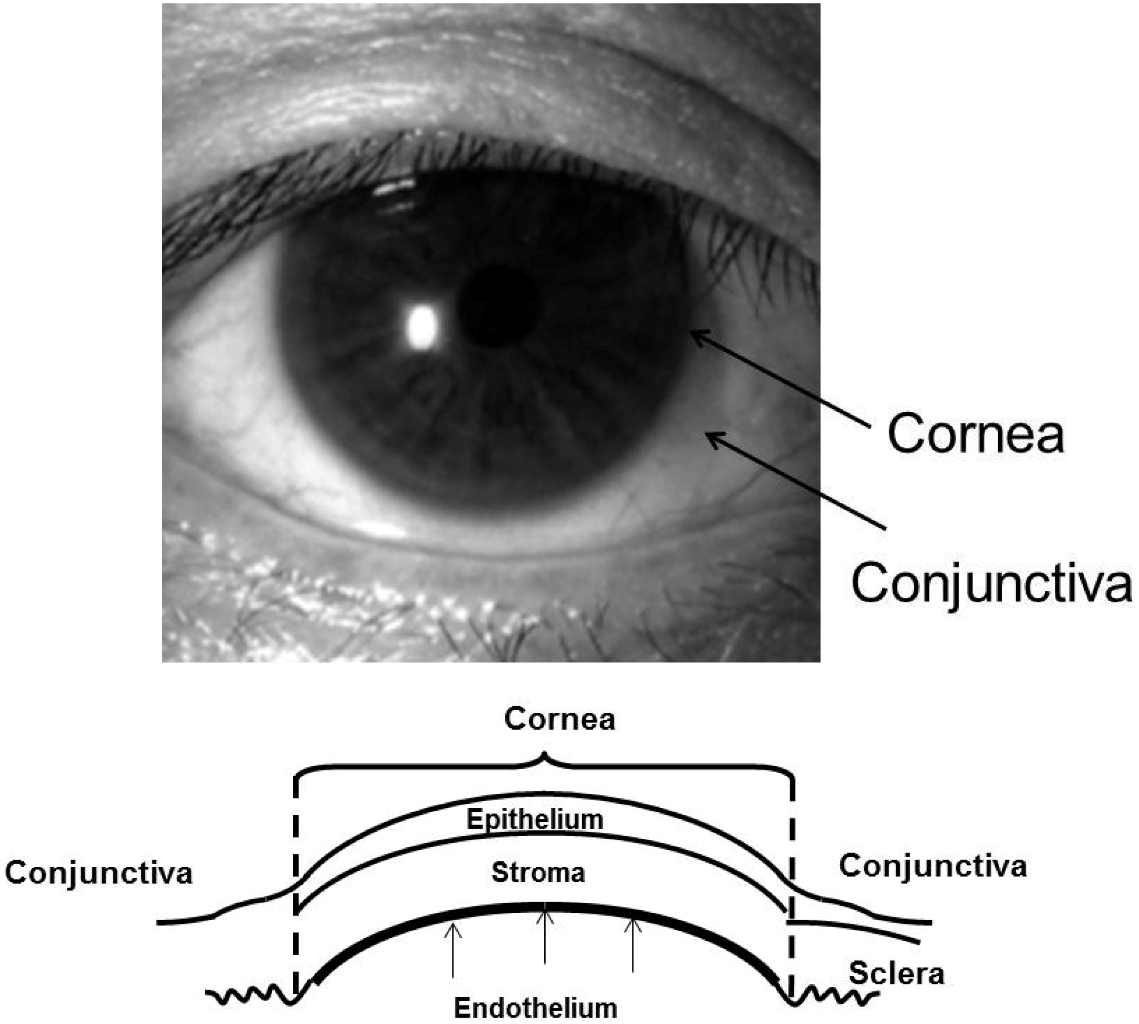
2. Culture Technique of Human Corneal Endothelial Cells
2.1. Extracellular Matrix Substrates for Human Corneal Endothelial Cell Culture
2.2. Growth Factors and Cytokines for Human Corneal Endothelial Cell Culture
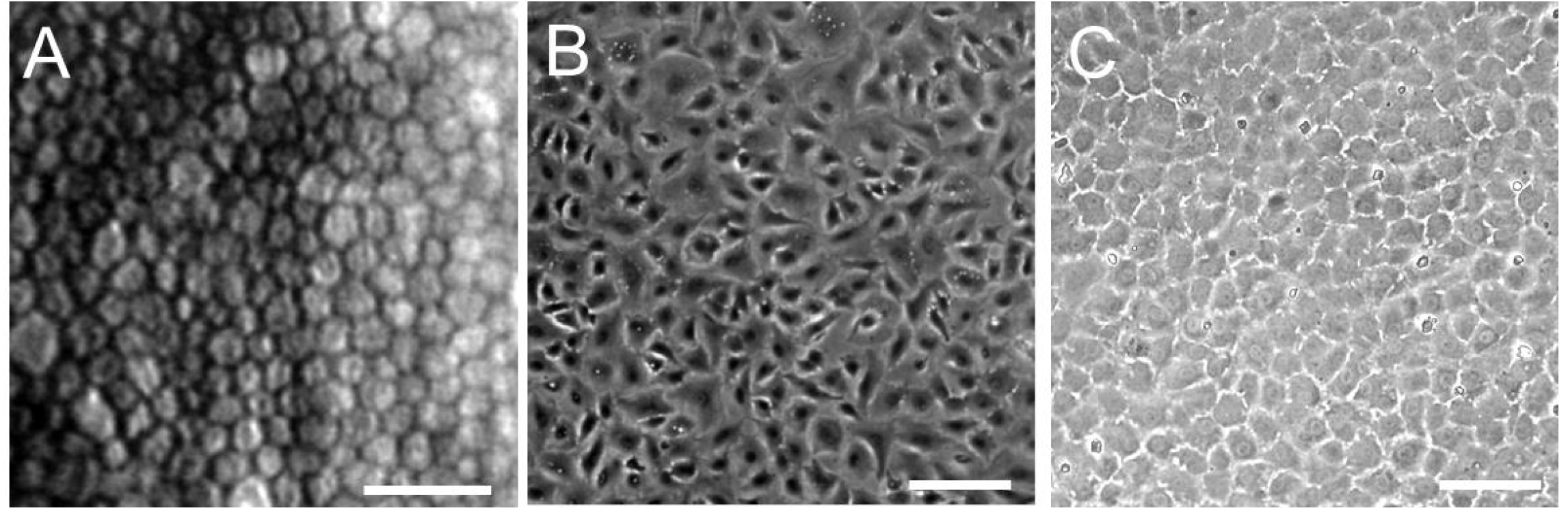
2.3. Overview of Human Corneal Endothelial Cell Culture
2.4. Human Corneal Endothelial Cell Culture in Our Laboratory
3. Construction of a Human Corneal Endothelial Cell Sheet
3.1. Reconstruction of a Penetrating Keratoplasty Graft Using Cultured Human Corneal Endothelial Cells
3.2. Construction of a Human Corneal Endothelial Cell Sheet for a Descemet Stripping Automated Endothelial Keratoplasty Graft
3.3. Density of Cultured Human Corneal Endothelial Cells on Collagen Sheets
3.4. Transport Activity of Human Corneal Endothelial Cell Sheets

4. Transplantation of Descemet Stripping Automated Endothelial Keratoplasty Grafts Using Cultured Human Corneal Endothelial Cells
4.1. Transplantation of Human Corneal Endothelial Cell Sheets Using Collagen as a Carrier in a Rabbit Model
4.2. Other Attempts to Transplant Cultured Corneal Endothelial Cell Sheets in Animal Models
| Author | Species of cultured CEC | Cell carrier | Host animal | Transplantation technique | Journal (Year) |
|---|---|---|---|---|---|
| Mimura | Human | Collagen | Rabbit | DSAEK | Invest Ophthalmol Vis Sci (2004) |
| Ishino | Human | Amniotic membrane | Rabbit | PKP | Invest Ophthalmol Vis Sci (2004) |
| Sumide | Human | PNIPAAm | Rabbit | PKP | FASEB J (2006) |
| Lai | Human | PNIPAAm and gelatin | Rabbit | DSAEK | Transplantation (2007) |
| Koizumi | Monkey | Collagen | Monkey | DSAEK | Invest Ophthalmol Vis Sci (2007) |
| Honda | Human | Thin human corneal stromal disc | Rabbit | DSAEK | Arch Ophthalmol (2009) |
4.3. Transplantation Technique of Human Corneal Endothelial Cell Sheets with Descemet Stripping Automated Endothelial Keratoplasty

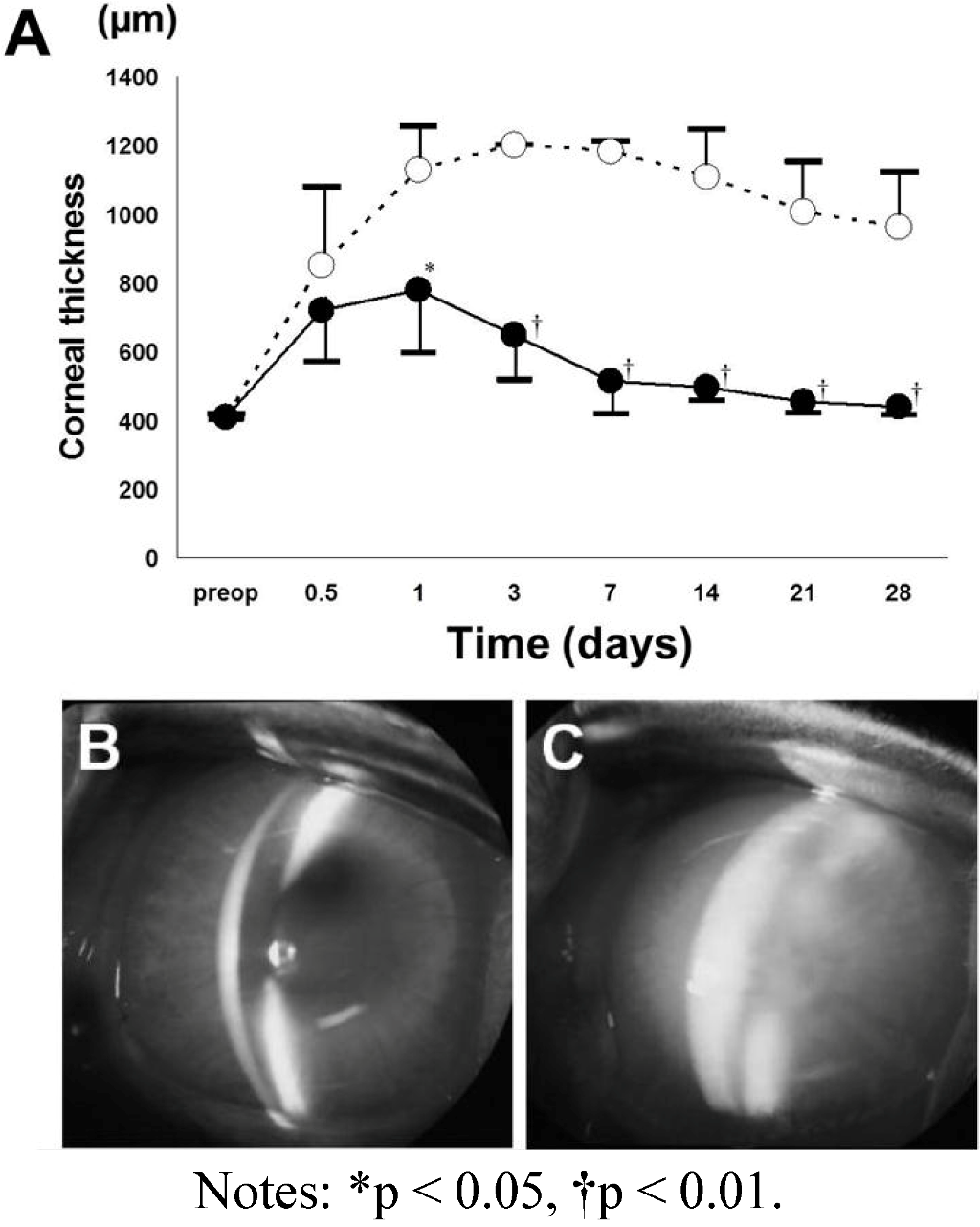
4.4. Observation after Transplantation
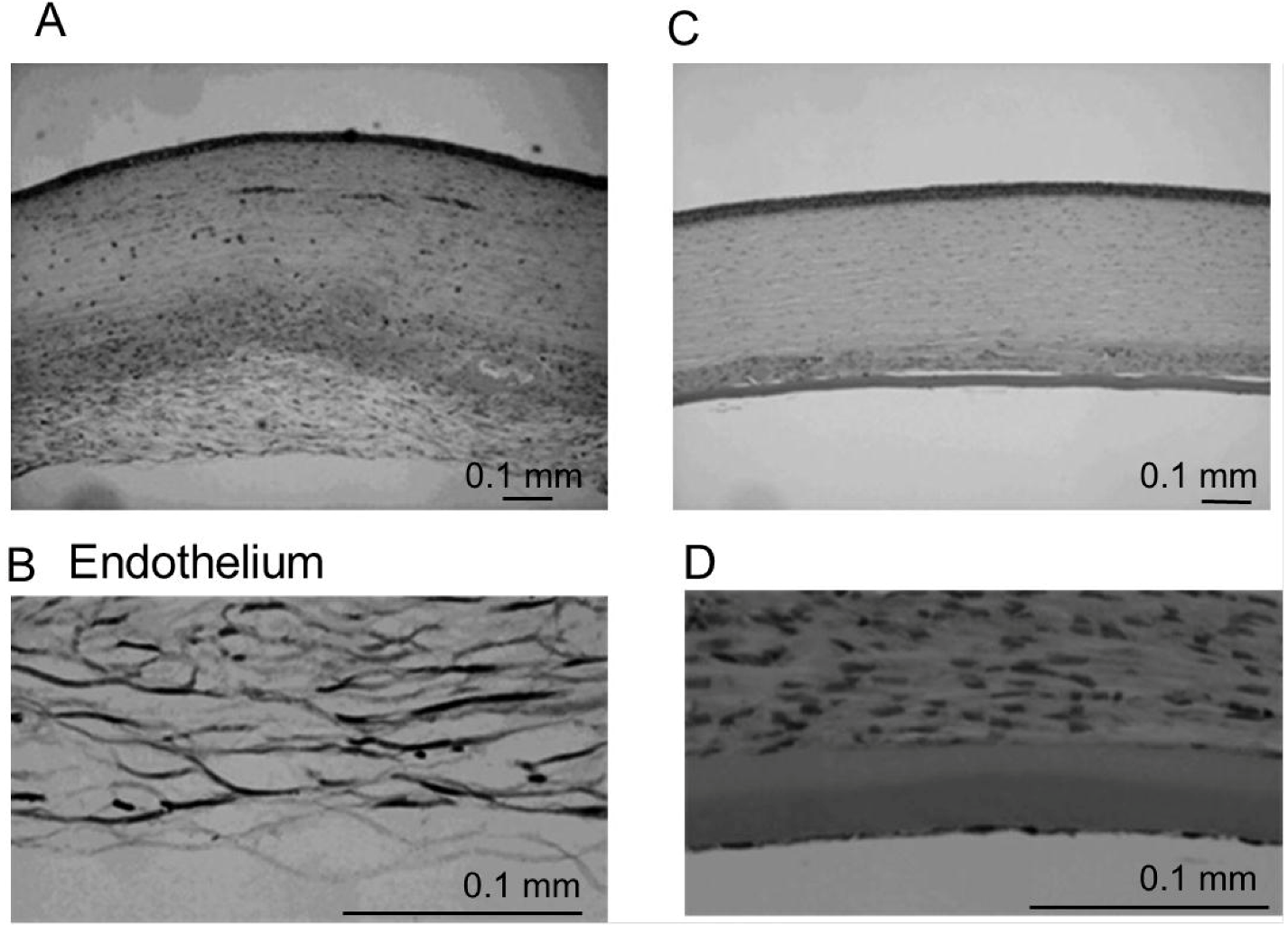
4.5. Histologic Examination
5. Conclusion
Acknowledgements
Financial Support
References
- Johnston, M.C.; Noden, D.M.; Hazelton, R.D.; Coulombre, J.L.; Coulombre, A.J. Origins of avian ocular and periocular tissues. Exp. Eye Res. 1979, 29, 27–43. [Google Scholar] [CrossRef]
- Bahn, C.F.; Falls, H.F.; Varley, G.A.; Meyer, R.F.; Edelhauser, H.F.; Bourne, W.M. Classification of corneal endothelial disorders based on neural crest origin. Ophthalmology 1984, 91, 558–563. [Google Scholar]
- Wilson, S.E.; Lloyd, S.A.; He, Y.G.; McCash, C.S. Extended life of human corneal endothelial cells transfected with the sv40 large t antigen. Invest. Ophthalmol. Vis. Sci. 1993, 34, 2112–2123. [Google Scholar]
- Wilson, S.E.; Weng, J.; Blair, S.; He, Y.G.; Lloyd, S. Expression of E6/E7 or SV40 large T antigen-coding oncogenes in human corneal endothelial cells indicates regulated high-proliferative capacity. Invest. Ophthalmol. Vis. Sci. 1995, 36, 32–40. [Google Scholar]
- Egan, C.A.; Savre-Train, I.; Shay, J.W.; Wilson, S.E.; Bourne, W.M. Analysis of telomere lengths in human corneal endothelial cells from donors of different ages. Invest. Ophthalmol. Vis. Sci. 1998, 39, 648–653. [Google Scholar]
- Senoo, T.; Joyce, N.C. Cell cycle kinetics in corneal endothelium from old and young donors. Invest. Ophthalmol. Vis. Sci. 2000, 41, 660–667. [Google Scholar]
- Senoo, T.; Obara, Y.; Joyce, N.C. EDTA: A promoter of proliferation in human corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2000, 41, 2930–2935. [Google Scholar]
- Joyce, N.C.; Meklir, B.; Joyce, S.J.; Zieske, J.D. Cell cycle protein expression and proliferative status in human corneal cells. Invest. Ophthalmol. Vis. Sci. 1996, 37, 645–655. [Google Scholar]
- Joyce, N.C.; Navon, S.E.; Roy, S.; Zieske, J.D. Expression of cell cycle-associated proteins in human and rabbit corneal endothelium in situ. Invest. Ophthalmol. Vis. Sci. 1996, 37, 1566–1575. [Google Scholar]
- Murphy, C.; Alvarado, J.; Juster, R.; Maglio, M. Prenatal and postnatal cellularity of the human corneal endothelium. A quanatitative histologic study. Invest. Ophthalmol. Vis. Sci. 1984, 25, 312–322. [Google Scholar]
- Bourne, W.M.; Nelson, L.R.; Hodge, D.O. Central corneal endothelial cell changes over a ten-year period. Invest. Ophthalmol. Vis. Sci. 1997, 38, 779–782. [Google Scholar]
- Hollingsworth, J.; Perez-Gomez, I.; Mutalib, H.A.; Efron, N. A population study of the normal cornea using an in vivo, slit-scanning confocal microscope. Optom. Vis. Sci. 2001, 78, 706–711. [Google Scholar] [CrossRef]
- Gorovoy, M.S. Descemet-stripping automated endothelial keratoplasty. Cornea 2006, 25, 886–889. [Google Scholar] [CrossRef]
- Koenig, S.B.; Covert, D.J. Early results of small-incision descemet’s stripping and automated endothelial keratoplasty. Ophthalmology 2007, 114, 221–226. [Google Scholar]
- Price, M.O.; Baig, K.M.; Brubaker, J.W.; Price, F.W., Jr. Randomized, prospective comparison of precut vs. surgeon-dissected grafts for descemet stripping automated endothelial keratoplasty. Am. J. Ophthalmol. 2008, 146, 36–41. [Google Scholar] [CrossRef]
- Terry, M.A.; Shamie, N.; Chen, E.S.; Hoar, K.L.; Friend, D.J. Endothelial keratoplasty a simplified technique to minimize graft dislocation, iatrogenic graft failure, and pupillary block. Ophthalmology 2008, 115, 1179–1186. [Google Scholar] [CrossRef]
- Insler, M.S.; Lopez, J.G. Transplantation of cultured human neonatal corneal endothelium. Curr. Eye Res. 1986, 5, 967–972. [Google Scholar] [CrossRef]
- Insler, M.S.; Lopez, J.G. Extended incubation times improve corneal endothelial cell transplantation success. Invest. Ophthalmol. Vis. Sci. 1991, 32, 1828–1836. [Google Scholar]
- Insler, M.S.; Lopez, J.G. Heterologous transplantation versus enhancement of human corneal endothelium. Cornea 1991, 10, 136–148. [Google Scholar] [CrossRef]
- Aboalchamat, B.; Engelmann, K.; Bohnke, M.; Eggli, P.; Bednarz, J. Morphological and functional analysis of immortalized human corneal endothelial cells after transplantation. Exp. Eye Res. 1999, 69, 547–553. [Google Scholar] [CrossRef]
- Engelmann, K.; Friedl, P. Optimization of culture conditions for human corneal endothelial cells. In Vitro Cell Dev. Biol. 1989, 25, 1065–1072. [Google Scholar] [CrossRef]
- Engelmann, K.; Drexler, D.; Bohnke, M. Transplantation of adult human or porcine corneal endothelial cells onto human recipients in vitro. Part I: Cell culturing and transplantation procedure. Cornea 1999, 18, 199–206. [Google Scholar] [CrossRef]
- Bohnke, M.; Eggli, P.; Engelmann, K. Transplantation of cultured adult human or porcine corneal endothelial cells onto human recipients in vitro. Part II: Evaluation in the scanning electron microscope. Cornea 1999, 18, 207–213. [Google Scholar] [CrossRef]
- Chen, K.H.; Azar, D.; Joyce, N.C. Transplantation of adult human corneal endothelium ex vivo: A morphologic study. Cornea 2001, 20, 731–737. [Google Scholar] [CrossRef]
- Amano, S. Transplantation of corneal endothelial cells. Nihon Ganka Gakkai Zasshi 2002, 106, 805–835. [Google Scholar]
- Amano, S. Transplantation of cultured human corneal endothelial cells. Cornea 2003, 22, S66–S74. [Google Scholar] [CrossRef]
- Mimura, T.; Amano, S.; Usui, T.; Araie, M.; Ono, K.; Akihiro, H.; Yokoo, S.; Yamagami, S. Transplantation of corneas reconstructed with cultured adult human corneal endothelial cells in nude rats. Exp. Eye Res. 2004, 79, 231–237. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Usui, T.; Tanaka, K.; Hattori, S.; Irie, S.; Miyata, K.; Araie, M.; Amano, S. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2992–2997. [Google Scholar]
- Ishino, Y.; Sano, Y.; Nakamura, T.; Connon, C.J.; Rigby, H.; Fullwood, N.J.; Kinoshita, S. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest. Ophthalmol. Vis. Sci. 2004, 45, 800–806. [Google Scholar] [CrossRef]
- Honda, N.; Mimura, T.; Usui, T.; Amano, S. Descemet stripping automated endothelial keratoplasty using cultured corneal endothelial cells in a rabbit model. Arch. Ophthalmol. 2009, 127, 1321–1326. [Google Scholar] [CrossRef]
- Choi, J.S.; Williams, J.K.; Greven, M.; Walter, K.A.; Laber, P.W.; Khang, G.; Soker, S. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials 2010, 31, 6738–6745. [Google Scholar] [CrossRef]
- Lai, J.Y.; Chen, K.H.; Hsiue, G.H. Tissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterials. Transplantation 2007, 84, 1222–1232. [Google Scholar] [CrossRef]
- Watanabe, R.; Hayashi, R.; Kimura, Y.; Tanaka, Y.; Kageyama, T.; Hara, S.; Tabata, Y.; Nishida, K. A novel gelatin hydrogel carrier sheet for corneal endothelial transplantation. Tissue Eng. Part A 2011, 17, 2213–2219. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Zhao, W.; Rong, M.; Li, H. Fabrication and characters of a corneal endothelial cells scaffold based on chitosan. J. Mater. Sci. Mater. Med. 2011, 22, 175–183. [Google Scholar] [CrossRef]
- Yue, B.Y.; Sugar, J.; Gilboy, J.E.; Elvart, J.L. Growth of human corneal endothelial cells in culture. Invest. Ophthalmol. Vis. Sci. 1989, 30, 248–253. [Google Scholar]
- Miyata, K.; Drake, J.; Osakabe, Y.; Hosokawa, Y.; Hwang, D.; Soya, K.; Oshika, T.; Amano, S. Effect of donor age on morphologic variation of cultured human corneal endothelial cells. Cornea 2001, 20, 59–63. [Google Scholar] [CrossRef]
- Engelmann, K.; Bohnke, M.; Friedl, P. Isolation and long-term cultivation of human corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 1988, 29, 1656–1662. [Google Scholar]
- Yamaguchi, M.; Ebihara, N.; Shima, N.; Kimoto, M.; Funaki, T.; Yokoo, S.; Murakami, A.; Yamagami, S. Adhesion, migration, and proliferation of cultured human corneal endothelial cells by laminin-5. Invest. Ophthalmol. Vis. Sci. 2011, 52, 679–684. [Google Scholar] [CrossRef]
- Blake, D.A.; Yu, H.; Young, D.L.; Caldwell, D.R. Matrix stimulates the proliferation of human corneal endothelial cells in culture. Invest. Ophthalmol. Vis. Sci. 1997, 38, 1119–1129. [Google Scholar]
- Joyce, N.C.; Zhu, C.C. Human corneal endothelial cell proliferation: Potential for use in regenerative medicine. Cornea 2004, 23, S8–S19. [Google Scholar] [CrossRef]
- Engelmann, K.; Friedl, P. Growth of human corneal endothelial cells in a serum-reduced medium. Cornea 1995, 14, 62–70. [Google Scholar]
- Samples, J.R.; Binder, P.S.; Nayak, S.K. Propagation of human corneal endothelium in vitro effect of growth factors. Exp. Eye Res. 1991, 52, 121–128. [Google Scholar]
- Schultz, G.; Cipolla, L.; Whitehouse, A.; Eiferman, R.; Woost, P.; Jumblatt, M. Growth factors and corneal endothelial cells: Iii. Stimulation of adult human corneal endothelial cell mitosis in vitro by defined mitogenic agents. Cornea 1992, 11, 20–27. [Google Scholar] [CrossRef]
- Shima, N.; Kimoto, M.; Yamaguchi, M.; Yamagami, S. Increased proliferation and replicative lifespan of isolated human corneal endothelial cells with l-ascorbic acid 2-phosphate. Invest. Ophthalmol. Vis. Sci. 2011, 52, 8711–8717. [Google Scholar] [CrossRef]
- Joyce, N.C.; Zhu, C.C.; Harris, D.L. Relationship among oxidative stress, DNA damage, and proliferative capacity in human corneal endotheliu. Invest. Ophthalmol. Vis. Sci. 2009, 50, 2116–2122. [Google Scholar] [CrossRef]
- Chen, Q.; Fischer, A.; Reagan, J.D.; Yan, L.J.; Ames, B.N. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl. Acad. Sci. USA. 1995, 92, 4337–4341. [Google Scholar]
- Miyashita, H.; Higa, K.; Kato, N.; Kawakita, T.; Yoshida, S.; Tsubota, K.; Shimmura, S. Hypoxia enhances the expansion of human limbal epithelial progenitor cells in vitro. Invest. Ophthalmol. Vis. Sci. 2007, 48, 3586–3593. [Google Scholar]
- Hansen, J.M.; Klass, M.; Harris, C.; Csete, M. A reducing redox environment promotes C2C12 myogenesis: Implications for regeneration in aged muscle. Cell Biol. Int. 2007, 31, 546–553. [Google Scholar]
- Lees, S.J.; Childs, T.E.; Booth, F.W. p21(Cip1) expression is increased in ambient oxygen, compared to estimated physiological (5%) levels in rat muscle precursor cell culture. Cell Prolif. 2008, 41, 193–207. [Google Scholar] [CrossRef]
- Mergler, S.; Valtink, M.; Coulson-Thomas, V.J.; Lindemann, D.; Reinach, P.S.; Engelmann, K.; Pleyer, U. TRPV channels mediate temperature-sensing in human corneal endothelial cells. Exp. Eye Res. 2010, 90, 758–770. [Google Scholar]
- Mergler, S.; Pleyer, U. Physiology of the human corneal endothelium--new insights from electrophysiological investigations. Klin. Monbl. Augenheilkd. 2011, 228, 520–524. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Toh, K.P.; Wu, F.Y.; Tan, D.T.; Mehta, J.S. Cultivation of human corneal endothelial cells isolated from paired donor corneas. PLoS ONE 2011, 6, e28310:1–e28310:10. [Google Scholar]
- Jackel, T.; Knels, L.; Valtink, M.; Funk, R.H.; Engelmann, K. Serum-free corneal organ culture medium (SFM) but not conventional minimal essential organ culture medium (MEM) protects human corneal endothelial cells from apoptotic and necrotic cell death. Br. J. Ophthalmol. 2011, 95, 123–130. [Google Scholar] [CrossRef]
- Zhu, C.; Joyce, N.C. Proliferative response of corneal endothelial cells from young and older donors. Invest. Ophthalmol. Vis. Sci. 2004, 45, 1743–1751. [Google Scholar] [CrossRef]
- Enomoto, K.; Mimura, T.; Harris, D.L.; Joyce, N.C. Age differences in cyclin-dependent kinase inhibitor expression and rb hyperphosphorylation in human corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2006, 47, 4330–4340. [Google Scholar] [CrossRef]
- Gospodarowicz, D.; Greenburg, G.; Alvarado, J. Transplantation of cultured bovine corneal endothelial cells to species with nonregenerative endothelium. The cat as an experimental model. Arch. Ophthalmol. 1979, 97, 2163–2169. [Google Scholar]
- Gospodarowicz, D.; Greenburg, G.; Alvarado, J. Transplantation of cultured bovine corneal endothelial cells to rabbit cornea: Clinical implications for human studies. Proc. Natl. Acad. Sci. USA 1979, 76, 464–468. [Google Scholar] [CrossRef]
- Gospodarowicz, D.; Greenburg, G. The coating of bovine and rabbit corneas denuded of their endothelium with bovine corneal endothelial cells. Exp. Eye Res. 1979, 28, 249–265. [Google Scholar]
- Jumblatt, M.M.; Maurice, D.M.; McCulley, J.P. Transplantation of tissue-cultured corneal endothelium. Invest. Ophthalmol. Vis. Sci. 1978, 17, 1135–1141. [Google Scholar]
- Lange, T.M.; Wood, T.O.; McLaughlin, B.J. Corneal endothelial cell transplantation using descemet’s membrane as a carrier. J. Cataract Refract. Surg. 1993, 19, 232–235. [Google Scholar]
- Joo, C.K.; Green, W.R.; Pepose, J.S.; Fleming, T.P. Repopulation of denuded murine descemet’s membrane with life-extended murine corneal endothelial cells as a model for corneal cell transplantation. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 174–180. [Google Scholar]
- McCulley, J.P.; Maurice, D.M.; Schwartz, B.D. Corneal endothelial transplantation. Ophthalmology 1980, 87, 194–201. [Google Scholar]
- Jumblatt, M.M.; Maurice, D.M.; Schwartz, B.D. A gelatin membrane substrate for the transplantation of tissue cultured cells. Transplantation 1980, 29, 498–499. [Google Scholar]
- Stenzel, K.H.; Dunn, M.W.; Rubin, A.L.; Miyata, T. Collagen gels: Design for a vitreous replace-ment. Science 1969, 164, 1282–1283. [Google Scholar]
- Hattori, S.; Adachi, E.; Ebihara, T.; Shirai, T.; Someki, I.; Irie, S. Alkali-treated collagen retained the triple helical conformation and the ligand activity for the cell adhesion via alpha2beta1 integrin. J. Biochem. 1999, 125, 676–684. [Google Scholar]
- Sumide, T.; Nishida, K.; Yamato, M.; Ide, T.; Hayashida, Y.; Watanabe, K.; Yang, J.; Kohno, C.; Kikuchi, A.; Maeda, N.; et al. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. FASEB J. 2006, 20, 392–394. [Google Scholar]
- Brown, S.I.; Hedbys, B.O. The effect of ouabain on the hydration of the cornea. Invest. Ophthalmol. 1965, 4, 216–221. [Google Scholar]
- Hodson, S. Evidence for a bicarbonate-dependent sodium pump in corneal endothelium. Exp. Eye Res. 1971, 11, 20–29. [Google Scholar]
- Huff, J.W.; Green, K. Demonstration of active sodium transport across the isolated rabbit corneal endothelium. Curr. Eye Res. 1981, 1, 113–114. [Google Scholar]
- Fischbarg, J.; Hernandez, J.; Liebovitch, L.S.; Koniarek, J.P. The mechanism of fluid and electrolyte transport across corneal endothelium: Critical revision and update of a model. Curr. Eye Res. 1985, 4, 351–360. [Google Scholar]
- Hodson, S.; Wigham, C. The permeability of rabbit and human corneal endothelium. J. Physiol. 1983, 342, 409–419. [Google Scholar]
- Wigham, C.; Hodson, S. The effect of bicarbonate ion concentration on trans-endothelial short circuit current in ox corneas. Curr. Eye Res. 1981, 1, 37–41. [Google Scholar] [CrossRef]
- Wigham, C.G.; Turner, H.C.; Swan, J.; Hodson, S.A. Modulation of corneal endothelial hydration control mechanisms by Rolipram. Pflugers Arch. 2000, 440, 866–870. [Google Scholar] [CrossRef]
- Koizumi, N.; Sakamoto, Y.; Okumura, N.; Okahara, N.; Tsuchiya, H.; Torii, R.; Cooper, L.J.; Ban, Y.; Tanioka, H.; Kinoshita, S. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest. Ophthalmol. Vis. Sci. 2007, 48, 4519–4526. [Google Scholar]
- Koizumi, N.; Sakamoto, Y.; Okumura, N.; Tsuchiya, H.; Torii, R.; Cooper, L.J.; Ban, Y.; Tanioka, H.; Kinoshita, S. Cultivated corneal endothelial transplantation in a primate: Possible future clinical application in corneal endothelial regenerative medicine. Cornea 2008, 27, S48–S55. [Google Scholar]
- Horan, P.K.; Melnicoff, M.J.; Jensen, B.D.; Slezak, S.E. Fluorescent cell labeling for in vivo and in vitro cell tracking. Methods Cell Biol. 1990, 33, 469–490. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Araie, M.; Amano, S. Comparison of rabbit corneal endothelial cell precursors in the central and peripheral cornea. Invest. Ophthalmol. Vis. Sci. 2005, 46, 3645–3648. [Google Scholar]
- Yamagami, S.; Yokoo, S.; Mimura, T.; Takato, T.; Araie, M.; Amano, S. Distribution of precursors in human corneal stromal cells and endothelial cells. Ophthalmology 2007, 114, 433–439. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mimura, T.; Yokoo, S.; Yamagami, S. Tissue Engineering of Corneal Endothelium. J. Funct. Biomater. 2012, 3, 726-744. https://doi.org/10.3390/jfb3040726
Mimura T, Yokoo S, Yamagami S. Tissue Engineering of Corneal Endothelium. Journal of Functional Biomaterials. 2012; 3(4):726-744. https://doi.org/10.3390/jfb3040726
Chicago/Turabian StyleMimura, Tatsuya, Seiichi Yokoo, and Satoru Yamagami. 2012. "Tissue Engineering of Corneal Endothelium" Journal of Functional Biomaterials 3, no. 4: 726-744. https://doi.org/10.3390/jfb3040726




