Novel in Vitro Model for Keratoconus Disease
Abstract
:1. Introduction
2. Methods
2.1. Primary Culture of Human Keratoconus (HKC) and Human Corneal Fibroblast Cells (HCF)
2.2. Assembly of Extracellular Matrix
2.3. Indirect-Immunofluorescence (IF)
2.4.Transmission Electron Microscopy (TEM)
2.5. Fast Fourier Transform Analysis (FFT)
2.6. 3D Cell Counting Software
2.7.Statistical Analysis
3. Results
3.1. Constructs Development

3.2. Ultrastructural Characterization
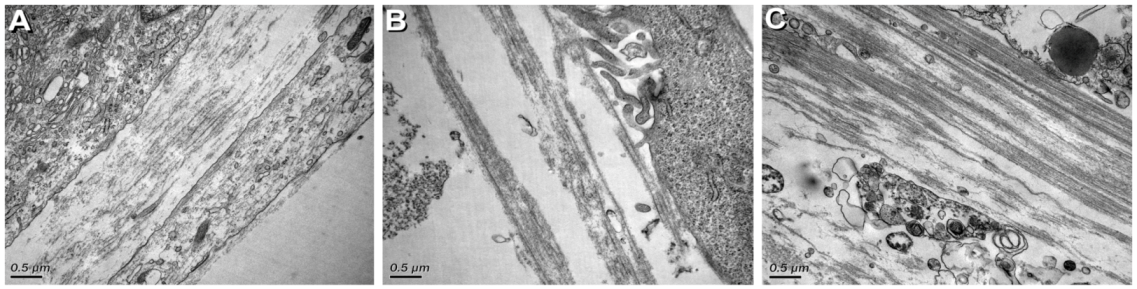
3.3. Specific ECM markers Expression
3.3.1. Type I and Type V Collagen (Col I and Col V)
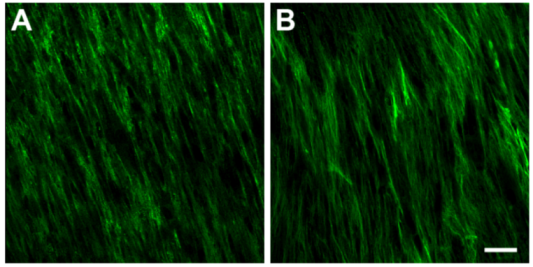
3.3.2. Type III Collagen (Col III)

3.3.3. Smooth Muscle Actin (SMA)

3.3.4. Fibronectin (EDA-Fn)

3.4. Fast Fourier Transform Analysis
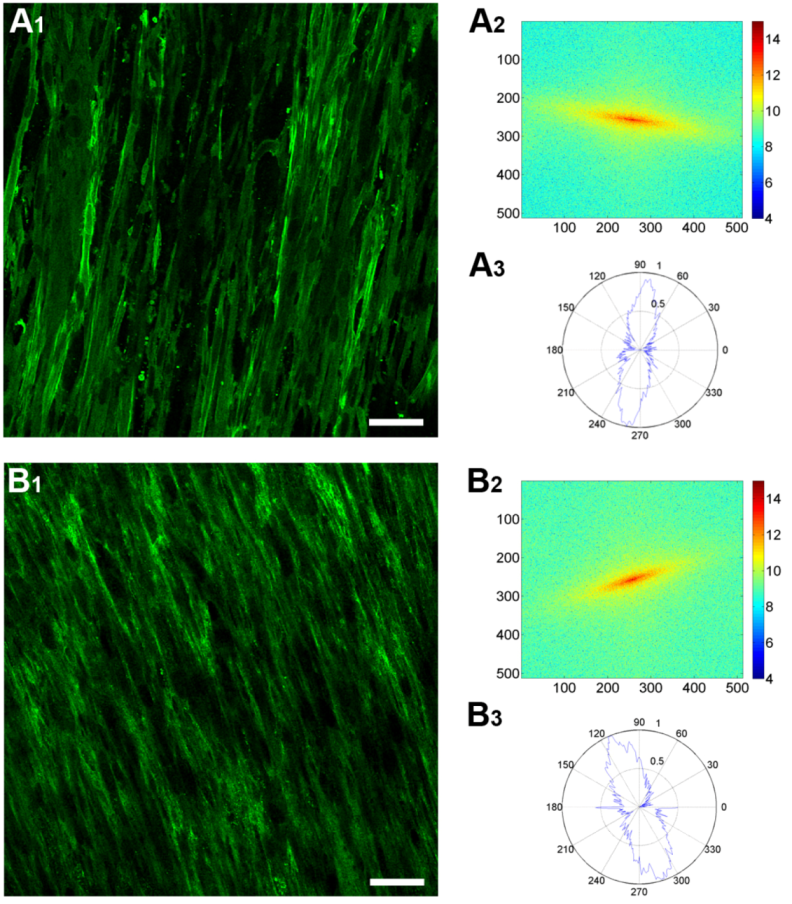
3.5. Cellular Alignment and Rotation

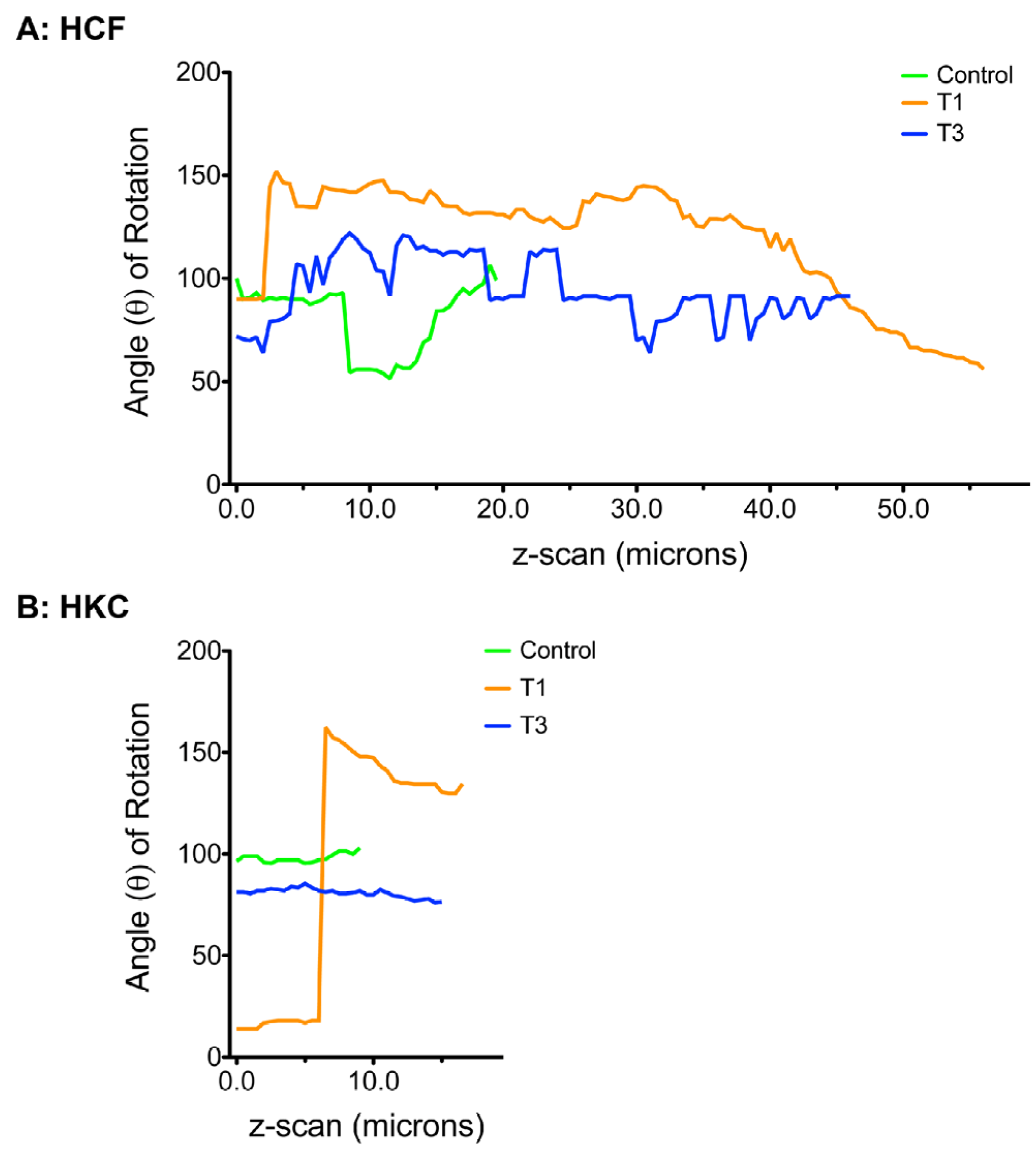
3.6. ECM Alignment and Rotation
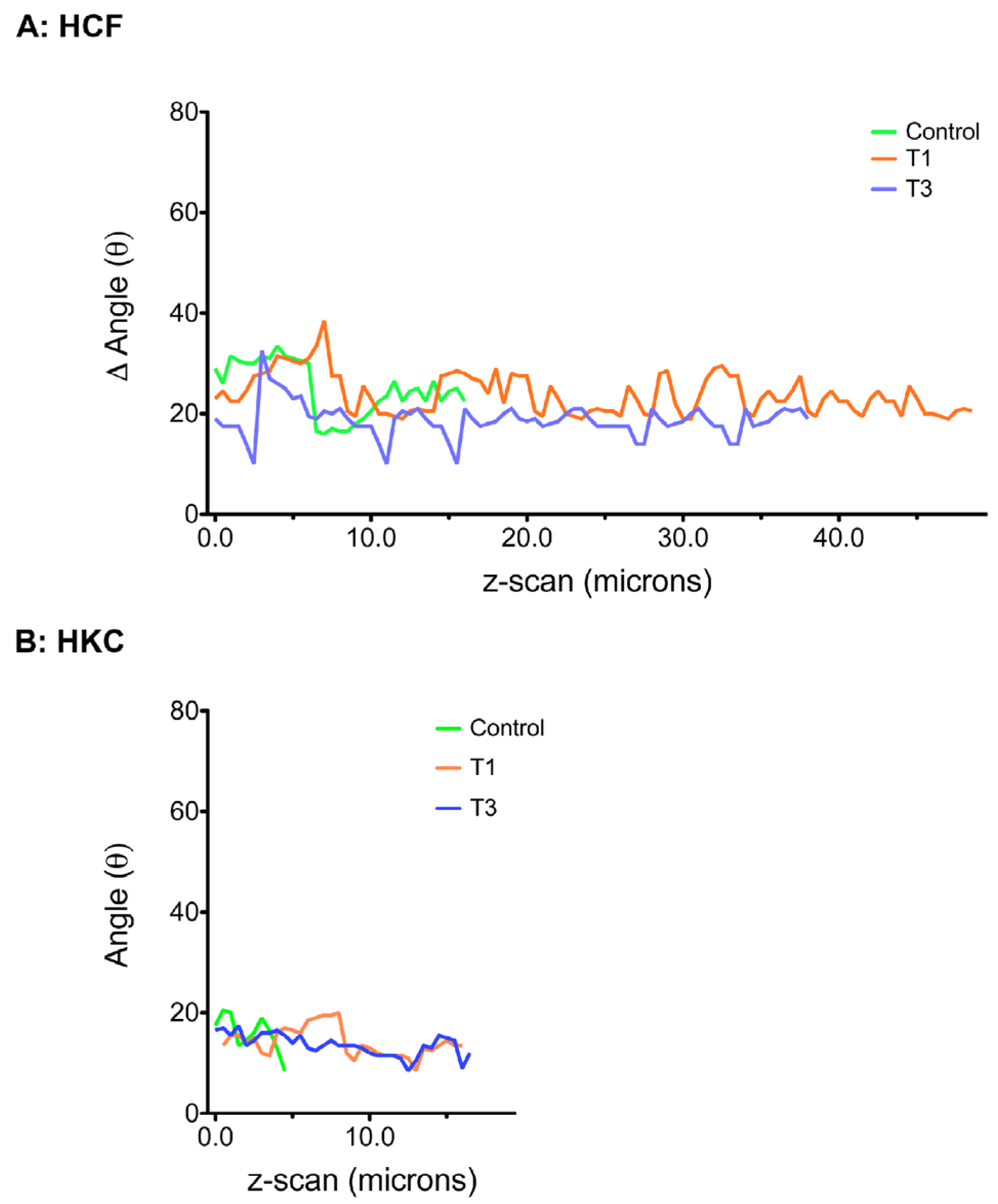

4. Discussion
5. Conclusions
Acknowledgments
References
- Maguire, L.J.; Bourne, W.M. Corneal topography of early keratoconus. Am. J. Ophthalmol. 1989, 108, 107–112. [Google Scholar]
- Arffa, R.C.; Grayson, M. Grayson’s Diseases of the Cornea, 4th ed; Mosby: St. Louis, MO, USA, 1997; p. 765. [Google Scholar]
- Kenney, M.C.; Brown, D. Research Overview. Available online: http://www.nkcf.org/en/research.html (accessed on 24 October 2012).
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Lowther, G. Epidemiology of Keratoconus. Available online: http://www.opt.indiana.edu/lowther/html/keratoconus_epidemiology.htm (accessed on 24 October 2012).
- Nielsen, K.; Hjortdal, J.; Aagaard, N.E.; Ehlers, N. Incidence and prevalence of keratoconus in Denmark. Acta Ophthalmol. Scand 2007, 85, 890–892. [Google Scholar] [CrossRef]
- Sharma, M.; Boxer Wachler, B.S. Comparison of single-segment and double-segment intacs for keratoconus and post-LASIK ectasia. Am. J. Ophthalmol. 2006, 141, 891–895. [Google Scholar] [CrossRef]
- Epstein, A.B. Keratoconus and Related Disorders. Available online: http://www.northshorecontactlens.com/Keratoconus.htm (accessed on 24 October 2012).
- Zieske, J.D.; Hutcheon, A.E.; Guo, X.; Chung, E.H.; Joyce, N.C. TGF-beta receptor types I and II are differentially expressed during corneal epithelial wound repair. Invest. Ophthalmol. Vis. Sci. 2001, 42, 1465–1471. [Google Scholar]
- Karamichos, D.; Guo, X.Q.; Hutcheon, A.E.; Zieske, J.D. Human corneal fibrosis: An in vitro model. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1382–1388. [Google Scholar] [CrossRef]
- Gipson, I.K.; Grill, S.M.; Spurr, S.J.; Brennan, S.J. Hemidesmosome formation in vitro. J. Cell. Biol. 1983, 97, 849–57. [Google Scholar] [CrossRef]
- Fung, D.T.; Sereysky, J.B.; Basta-Pljakic, J.; Laudier, D.M.; Huq, R.; Jepsen, K.J.; Schaffler, M.B.; Flatow, E.L. Second harmonic generation imaging and Fourier transform spectral analysis reveal damage in fatigue-loaded tendons. Ann. Biomed. Eng. 2010, 38, 1741–1751. [Google Scholar] [CrossRef]
- Sander, E.A; Barocas, V.H. Comparison of 2D fiber network orientation measurement methods. J. Biomed. Mater. Res. A 2009, 88, 322–331. [Google Scholar]
- Karamichos, D.; Hutcheon, A.E.; Zieske, J.D. Transforming growth factor-beta3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J. Tissue Eng. Regen. Med. 2011, 5, e228–e238. [Google Scholar] [CrossRef]
- Karamichos, D.; Rich, C.B.; Hutchen, A.E.K.; Ren, R.Y.; Saitta, B.; Trinkaus-Randall, V.; Zieske, J.D. Self-assembled matrix by umbilical cord stem cells. J. Funct. Biomater. 2011, 2, 213–229. [Google Scholar] [CrossRef]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell. Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Edwards, M.; McGhee, C.N.; Dean, S. The genetics of keratoconus. Clin. Exp. Ophthalmol. 2001, 29, 345–351. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Planck, S.T.; Huang, X.N.; Rich, L.; Ansel, J.C. Detection of mRNA for the cytokines, interleukin-1 alpha and interleukin-8, in corneas from patients with pseudophakic bullous keratopathy. Invest. Ophthalmol. Vis. Sci. 1995, 36, 2151–2155. [Google Scholar]
- Zhou, L.; Yue, B.Y.; Twining, S.S.; Sugar, J.; Feder, R.S. Expression of wound healing and stress-related proteins in keratoconus corneas. Curr. Eye Res. 1996, 15, 1124–1231. [Google Scholar] [CrossRef]
- Brunet, C.L.; Sharpe, P.M.; Ferguson, M.W. Inhibition of TGF-beta 3 (but not TGF-beta 1 or TGF-beta 2) activity prevents normal mouse embryonic palate fusion. Int. J. Dev. Biol. 1995, 39, 345–355. [Google Scholar]
- Krummel, T.M.; Michna, B.A.; Thomas, B.L.; Sporn, M.B.; Nelson, J.M.; Salzberg, A.M.; Cohen, I.K.; Diegelmann, R.F. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J. Pediatr. Surg. 1988, 23, 647–652. [Google Scholar]
- Shah, M.; Foreman, D.M.; Ferguson, M.W. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J. Cell. Sci. 1995, 108, 985–1002. [Google Scholar]
- Carrington, L.M.; Albon, J.; Anderson, I.; Kamma, C.; Boulton, M. Differential regulation of key stages in early corneal wound healing by TGF-beta isoforms and their inhibitors. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1886–1894. [Google Scholar] [CrossRef]
- Faouzi, S.; Le Bail, B.; Neaud, V.; Boussarie, L.; Saric, J.; Bioulac-Sage, P.; Balabaud, C.; Rosenbaum, J. Myofibroblasts are responsible for collagen synthesis in the stroma of human hepatocellular carcinoma: An in vivo and in vitro study. J. Hepatol. 1999, 30, 275–284. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Karamichos, D.; Zareian, R.; Guo, X.; Hutcheon, A.E.K.; Ruberti, J.W.; Zieske, J.D. Novel in Vitro Model for Keratoconus Disease. J. Funct. Biomater. 2012, 3, 760-775. https://doi.org/10.3390/jfb3040760
Karamichos D, Zareian R, Guo X, Hutcheon AEK, Ruberti JW, Zieske JD. Novel in Vitro Model for Keratoconus Disease. Journal of Functional Biomaterials. 2012; 3(4):760-775. https://doi.org/10.3390/jfb3040760
Chicago/Turabian StyleKaramichos, Dimitrios, Ramin Zareian, Xiaoqing Guo, Audrey E.K. Hutcheon, Jeffrey W. Ruberti, and James D. Zieske. 2012. "Novel in Vitro Model for Keratoconus Disease" Journal of Functional Biomaterials 3, no. 4: 760-775. https://doi.org/10.3390/jfb3040760




