Soft Polymers for Building up Small and Smallest Blood Supplying Systems by Stereolithography
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Prepolymers PTHF-DA 1-4
| PTHF-DA | Mn [g·mol−1] [a] | P [a] | n [a] | mp. [°C] [b] | viscosity [mPa·s] [c] |
|---|---|---|---|---|---|
| 1 | 1,050 | 1.67 | 13 | 5–12 | 27 |
| 2 | 2,544 | 1.99 | 34 | 22 | 151 |
| 3 | 3,856 | 1.99 | 52 | 26–37 | 314 |
| 4 | 4,936 | 2.36 | 67 | 40.5 | 932 |
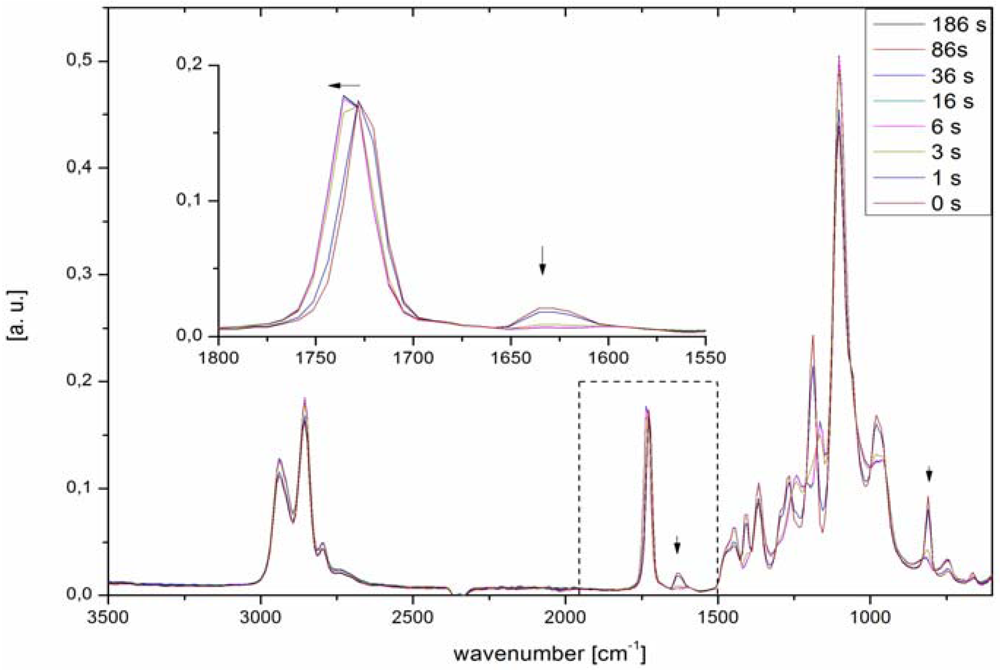
2.1.1. FTIR
2.2. Characterization of Polymers PTHF-PA 1–4
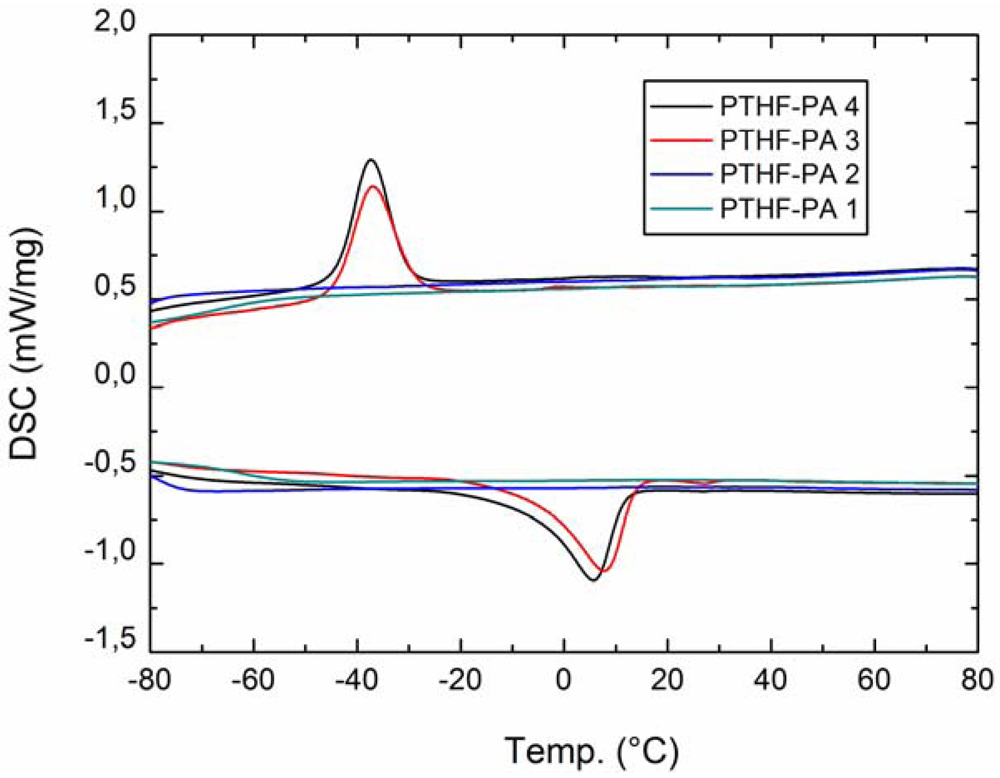
2.2.1. DSC
2.2.2. Mechanical Characterization
| PTHF-PA | swelling in H2O [%] | Contact angleadv [°] | Contact anglerec [°] | Young’s modulus [MPa] | Bending strength [MPa] | Tc/ΔHc [°C]/[J/g] | Tm/ΔHm [°C]/[J/g] | Gelation [%] |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.3 | 64.2 ± 5.8 | 39.2 ± 1.9 | 27.5 | 3.5 | - | - | 98.2 |
| 2 | 1.9 | 72.5 ± 3.4 | 50.1 ± 1.3 | 10.7 | 1.8 | - | - | 97.2 |
| 3 | 1.5 | 84.8 ± 8.0 | 42.4 ± 2.6 | 5.7 | 1.3 | −37.0/39.6 | 7.2/42.8 | 97.2 |
| 4 | 1.9 | 77.5 ± 2.8 | 44.0 ± 2.2 | 8.0 | 1.1 | −37.3/39.6 | 5.0/42.4 | 98.6 |
2.2.3. Shape Memory Effect
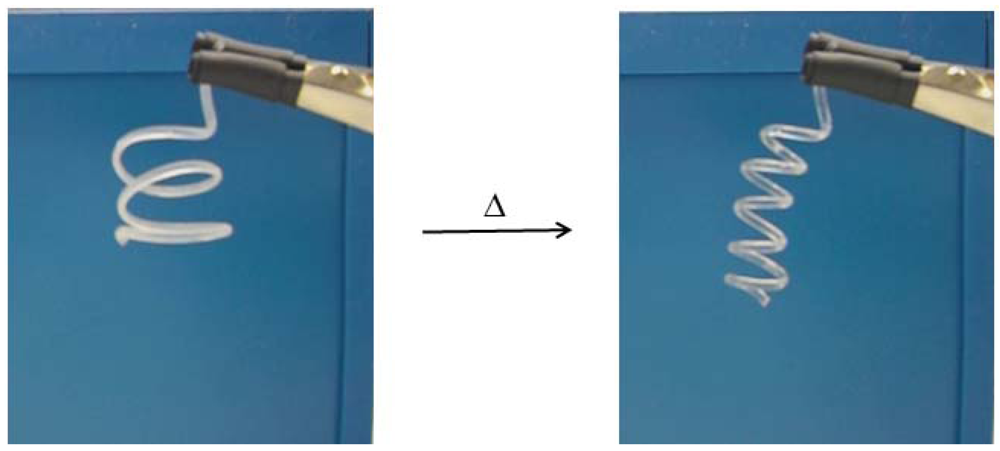
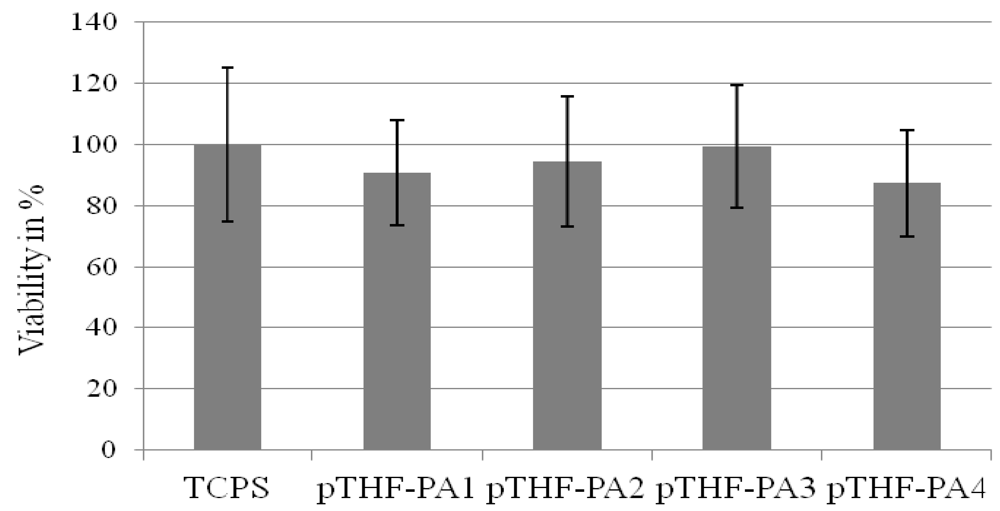
2.2.4. Biocompatibility
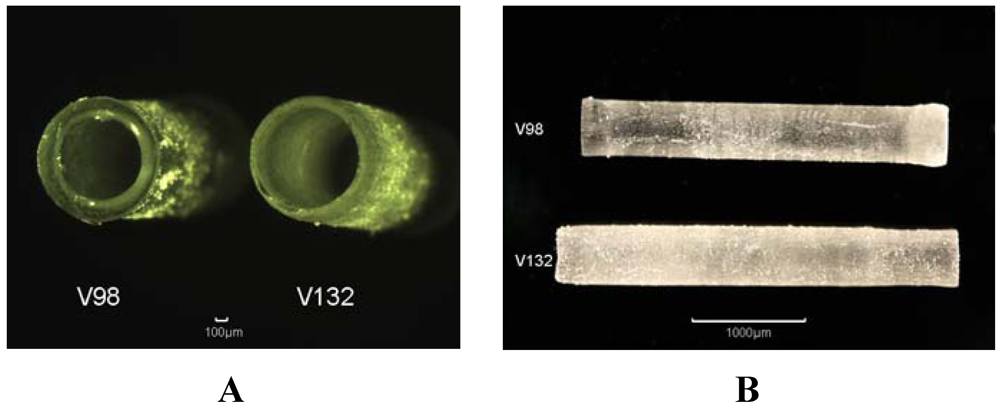
2.2.5. Stereolithography Processing (SL)
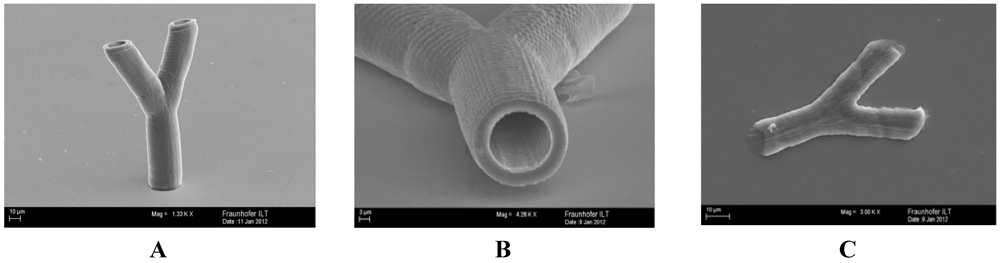
2.2.6. Multiphoton Polymerization (MPP)
3. Experimental Section
3.1. Instrumentation
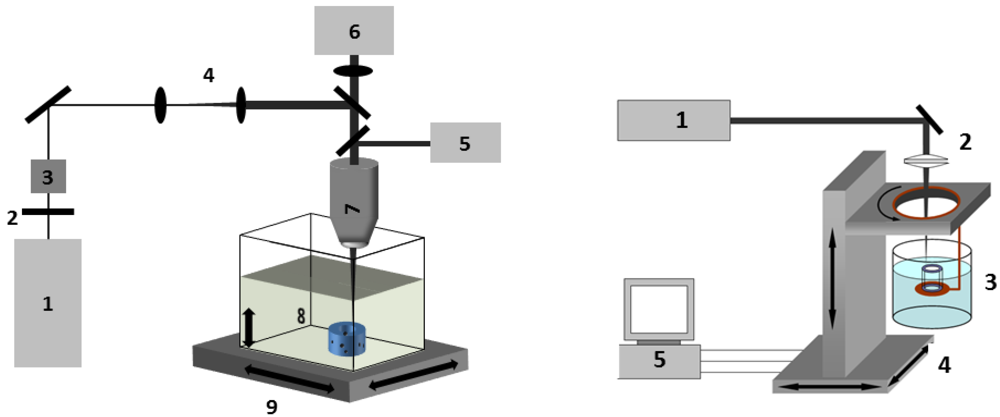
3.2. Processing Setup
4. Conclusions
Supplementary Files
Acknowledgments
References
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Advan. Drug Delivery Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef]
- Ju, Y.M.; Choi, J.S.; Atala, A.; Yoo, J.J.; Lee, S.J. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials 2010, 31, 4313–4321. [Google Scholar]
- Bellan, L.M.; Singh, S.P.; Henderson, P.W.; Porri, T.J.; Craighead, H.G.; Spector, J.A. Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter 2009, 5, 1354–1357. [Google Scholar] [CrossRef]
- Hieu, L.C.; Zlatov, N.; Sloten, J.V.; Bohez, E.; Khanh, L.; Binh, P.H.; Oris, P.; Toshev, Y. Medical rapid prototyping applications and methods. Assembly Automation 2005, 25, 284–292. [Google Scholar] [CrossRef]
- Petzold, R.; Zeilhofer, H.-F.; Kalender, W.A. Rapid prototyping technology in medicine—Basics and applications. Comput. Med. Imaging Graph. 1999, 23, 277–284. [Google Scholar] [CrossRef]
- Peltola, S.M.; Melchels, F.P.W.; Grijpma, D.W.; Kellomäki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Schuster, M.; Turecek, C.; Varga, F.; Lichtenegger, H.; Stampfl, J.; Liska, R. 3D-shaping of biodegradable photopolymers for hard tissue replacement. Appl. Surf. Sci. 2007, 254, 1131–1134. [Google Scholar] [CrossRef]
- Liska, R.; Schuster, M.; Inführ, R.; Turecek, C.; Fritscher, C.; Seidl, B.; Schmidt, V.; Kuna, L.; Haase, A.; Varga, F.; Lichtenegger, H.; Stampfl, J. Photopolymers for rapid prototyping. J. Coating. Technol. Res. 2007, 4, 505–510. [Google Scholar] [CrossRef]
- Heller, C.; Schuster, M.; Baudis, S.; Turecek, C.; Varga, F.; Bergmeister, H.; Weigel, G.; Stampfl, J.; Liska, R. New materials for 3D-scaffolds for tissue engineering applications. Tissue Eng. Part A 2008, 5, 761–761. [Google Scholar]
- Stampfl, J.; Baudis, S.; Heller, C.; Liska, R.; Neumeister, A.; Kling, R.; Ostendorf, A.; Spitzbart, M. Photopolymers with tunable mechanical properties processed by laser-based high-resolution stereolithography. J. Micromechanic. Microengineer. 2008, 18, 125014:1–125014:9. [Google Scholar]
- Schuster, M.; Turecek, C.; Kaiser, B.; Stampfl, J.; Liska, R.; Varga, F. Evaluation of biocompatible photopolymers I: Photoreactivity and mechanical properties of reactive diluents. J. Macromol. Sci. Part A 2007, 44, 547–557. [Google Scholar]
- Bens, A.; Seitz, H.; Bermes, G.; Emons, M.; Pansky, A.; Roitzheim, B.; Tobiasch, E.; Tille, C. Non-toxic flexible photopolymers for medical stereolithography technology. Rapid Prototyping J. 2007, 13, 38–47. [Google Scholar] [CrossRef]
- Baudis, S.; Heller, C.; Liska, R.; Stampfl, J.; Bergmeister, H.; Weigel, G. (Meth)acrylate-based photoelastomers as tailored biomaterials for artificial vascular grafts. J. Polym. Sci. 2009, 47, 2664–2676. [Google Scholar]
- Lillie, M.A.; Gosline, J.M. Limits to the durability of arterial elastic tissue. Biomaterials 2007, 28, 2021–2031. [Google Scholar] [CrossRef]
- Zilla, P.; Bezuidenhout, D.; Human, P. Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterials 2007, 28, 5009–5027. [Google Scholar] [CrossRef]
- Engelhardt, S.; Hoch, E.; Borchers, K.; Meyer, W.; Krüger, H.; Tovar, G.E.; Gillner, A. Fabrication of 2D protein microstructures and 3D polymer–protein hybrid microstructures by two-photon polymerization. Biofabrication 2011, 3, 025003:1–025003:9. [Google Scholar]
- Engelhardt, S.; Hu, Y.; Seiler, N.; Riester, D.; Meyer, W.; Krüger, H.; Wehner, M.; Bremer-Koebberling, E.; Gillner, A. 3D-microfabrication of polymer-protein hybrid structures with a Q-switched microlaser. JLMN 2011, 6, 54–58. [Google Scholar] [CrossRef]
- Novosel, E.C.; Meyer, W.; Klechowitz, N.; Krüger, H.; Wegener, M.; Walles, H.; Tovar, G.E.M.; Hirth, T.; Kluger, P.J. Evaluation of cell-material interactions on newly designed, printable polymers for tissue engineering applications. Adv. Eng. Mater. 2011, 13, B467–B475. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, W.; Wan, G.; Peng, Y. Polytetrahydrofuran amphiphilic networks. I. Synthesis and characterization of polytetrahydrofuran acrylate ditelechelic and polyacrylamide-<I>l</I>-polytetrahydrofuran networks. J. Polym Sci Part A 2000, 38, 3812–3820. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Meyer, W.; Engelhardt, S.; Novosel, E.; Elling, B.; Wegener, M.; Krüger, H. Soft Polymers for Building up Small and Smallest Blood Supplying Systems by Stereolithography. J. Funct. Biomater. 2012, 3, 257-268. https://doi.org/10.3390/jfb3020257
Meyer W, Engelhardt S, Novosel E, Elling B, Wegener M, Krüger H. Soft Polymers for Building up Small and Smallest Blood Supplying Systems by Stereolithography. Journal of Functional Biomaterials. 2012; 3(2):257-268. https://doi.org/10.3390/jfb3020257
Chicago/Turabian StyleMeyer, Wolfdietrich, Sascha Engelhardt, Esther Novosel, Burkhard Elling, Michael Wegener, and Hartmut Krüger. 2012. "Soft Polymers for Building up Small and Smallest Blood Supplying Systems by Stereolithography" Journal of Functional Biomaterials 3, no. 2: 257-268. https://doi.org/10.3390/jfb3020257







