Quercetin-Imprinted Nanospheres as Novel Drug Delivery Devices
Abstract
:1. Introduction
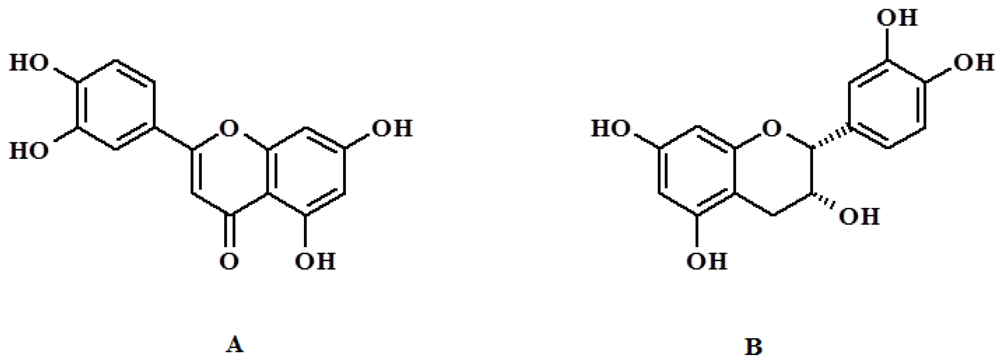
2. Results and Discussion
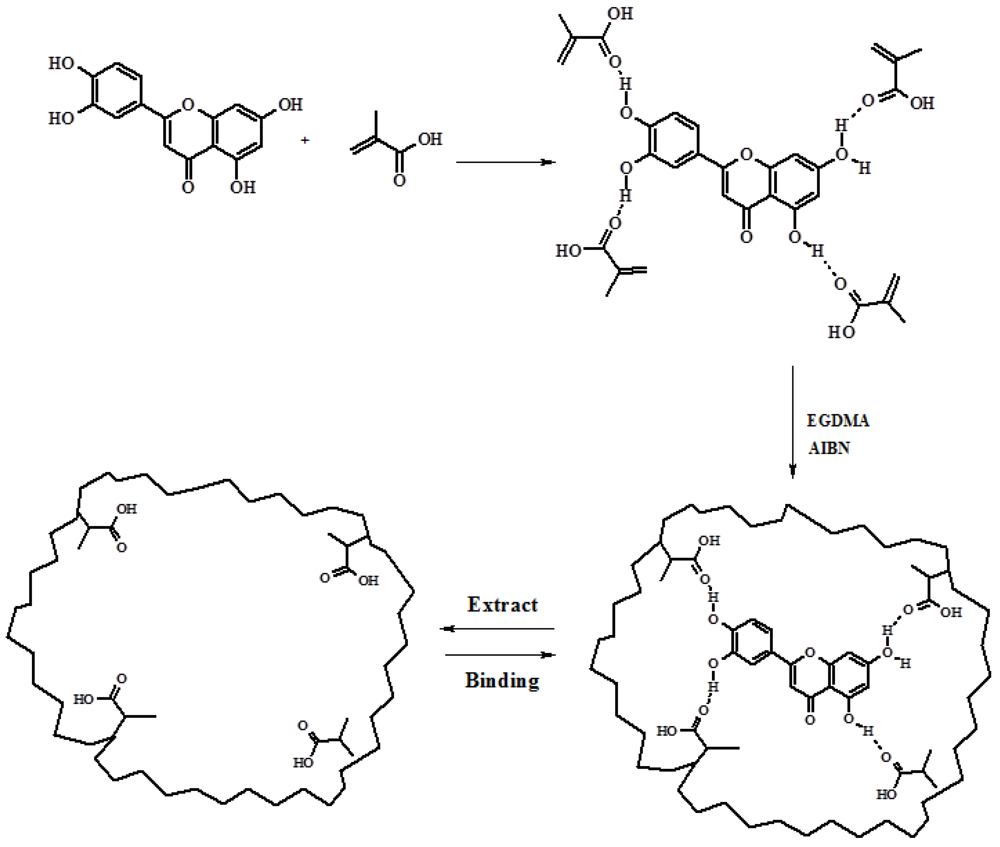
2.1. Synthesis and Characterization of QC Imprinted Polymers
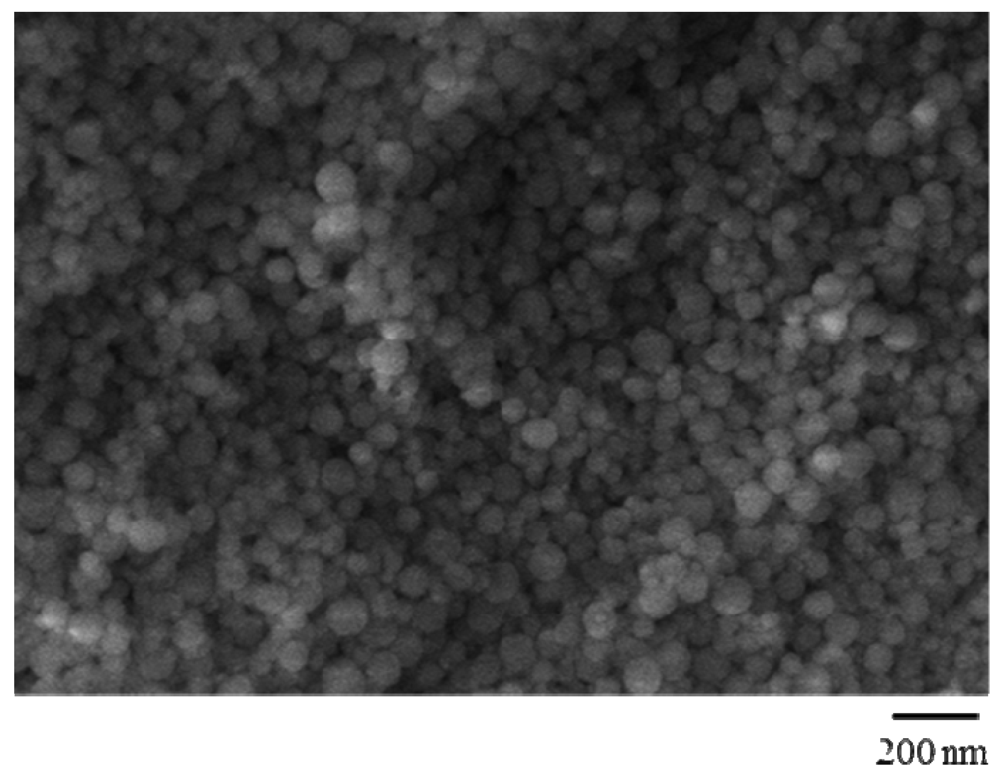
| Polymers | QC/MAA/EGDMA (mmol) | dn (nm) | Polydispersivity |
|---|---|---|---|
| MIP | 1.0/16.0/25.0 | 83 | 1.08 |
| NIP | --/16.0/25.0 | 87 | 1.06 |
| Matrix | % Q Bound | %CT Bound | αQ | αCT | Ε | Water Content (%) |
|---|---|---|---|---|---|---|
| MIP | 25 ± 1.1 | 14 ± 1.4 | 2.27 | 1.2 | 1.78 | 558 ± 0.6 |
| NIP | 11 ± 1.3 | 11 ± 1.1 | 535 ± 0.5 |
2.2. Evaluation of the Imprinting Effect: Binding Experiments in Water Media
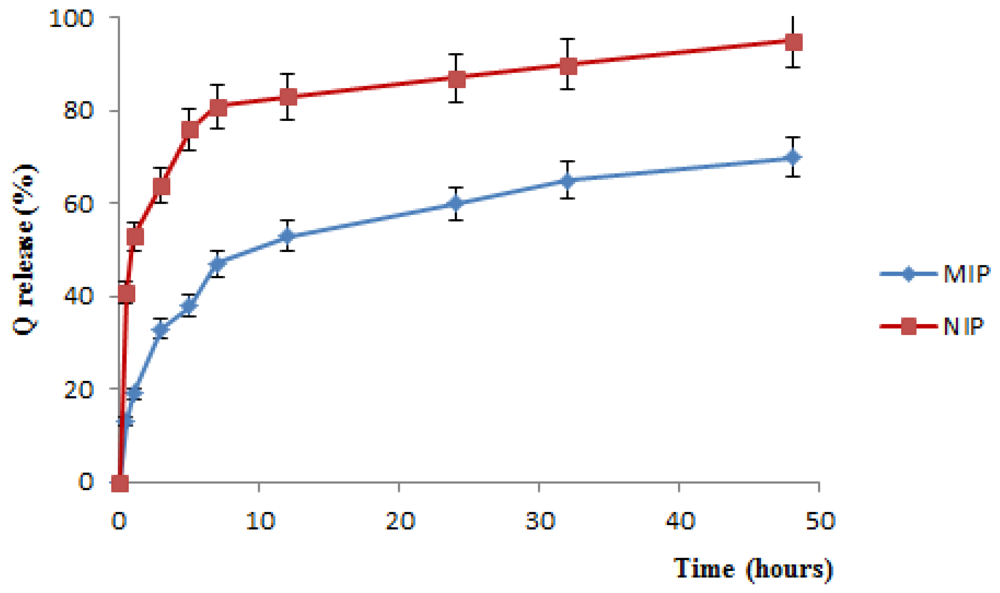
2.3. In Vitro Release Studies
2.4. Cytotoxicity Test

3. Experimental Section
3.1. Reagents and Standards
3.2. Synthesis of QC Imprinted Nanospheres
3.3. Water Content of Imprinted Polymers
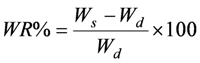
3.4. Binding Experiments
3.5. Drug Loading by the Soaking Procedure
3.6. In Vitro Release Studies
3.7. Cell Growth Inhibition Studies
3.8. HPLC Analysis
3.9. Scanning Electron Microscopy
3.10. Dimensional Analysis
4. Conclusions
Acknowledgments
References
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent design to treat complex disease. Pharm. Res. 2007, 23, 1417–1450. [Google Scholar] [CrossRef]
- Torchilin, V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007, 9, 128–147. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Chinsriwongkul, A.; Chareanputtakhun, P.; Ngawhirunpat, T.; Rojanarata, T.; Sila-On, W.; Rutkanonkai, U.; Opanasopit, P. Nanostructured Lipid Carriers (NLC) for parenteral delivery of ananticancer drug. AAPS PharmSciTech 2012, 13, 150–158. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Jain, N.K.; Diwan, P.V.; Khopade, A.J. Solubility enhancement of indomethacin with withpoly(amidoamine) dendrimersand targeting to inflammatory regions of arthritic rats. J. Drug Target. 2004, 12, 9–10. [Google Scholar]
- Byrne, M.E.; Salian, V. Molecular imprinting within hydrogels II: Progress and analysis of the field. Int. J. Pharm. 2004, 364, 188–212. [Google Scholar] [CrossRef]
- Lu, C.; Zhou, W.; Han, B.; Yang, H.; Chen, X.; Wang, X. Surface-imprinted core-shell nanoparticles for sorbent assays. Anal. Chem. 2007, 79, 5457–5461. [Google Scholar] [CrossRef]
- Xie, C.; Liu, B.; Wang, Z.; Gao, D.; Guan, G.; Zhang, Z. Molecular imprinting at walls of silica nanotubes for TNT recognition. Anal. Chem. 2008, 80, 437–443. [Google Scholar] [CrossRef]
- Cirillo, G.; Iemma, F.; Puoci, F.; Parisi, O.I.; Curcio, M.; Spizzirri, U.G.; Picci, N. Imprinted hydrophilic nanospheres as drug delivery systems for 5-fluorouracil sustained release. J. Drug Target. 2009, 17, 72–77. [Google Scholar] [CrossRef]
- Curcio, M.; Puoci, F.; Cirillo, G.; Iemma, F.; Spizzirri, U.G.; Picci, N. Selective determination of melamine in aqueous medium by molecularly imprinted solid phase extraction. J. Agric. Food Chem. 2010, 58, 11883–11887. [Google Scholar] [CrossRef]
- Vidyasankar, S.; Arnold, F.H. Molecular imprinting: selective materials for separations, sensors and catalysis. Curr. Opin. Biotechnol. 1995, 6, 218–224. [Google Scholar] [CrossRef]
- Li, P.; Huang, Y.; Hu, J.; Yuan, C.; Lin, B. Surface plasmon resonance studies on molecular imprinting. Sensors 2002, 2, 35–40. [Google Scholar] [CrossRef]
- Ansell, R.J.; Ramström, O.; Mosbach, K. Towards artificial antibodies prepared by molecular imprinting. Clin. Chem. 1996, 42, 1506–1512. [Google Scholar]
- Cirillo, G.; Curcio, M.; Parisi, O.I.; Puoci, F.; Iemma, F.; Spizzirri, U.G.; Picci, N. Gastro-intestinal sustainedreleaseofphyticacidbymolecularlyimprintedmicroparticles. Pharm. Dev. Technol. 2010, 15, 526–531. [Google Scholar] [CrossRef]
- Cunliffe, D.; Kirby, A.; Alexander, C. Molecularly imprinted drug delivery systems. Adv. Drug Deliv. Rev. 2005, 57, 1836–1853. [Google Scholar]
- Sellergren, B.; Allender, C.J. Molecularly imprinted polymers: A bridge to advanced drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1733–1741. [Google Scholar] [CrossRef]
- Singh, B.; Chauhan, N.; Sharma, V. Design of molecular imprinted hydrogels for controlled release of cisplatin: Evaluation of network density of hydrogels. Ind. Eng. Chem. Res. 2011, 50, 13742–13751. [Google Scholar]
- Alvarez-Lorenzo, C.; Yañez, F.; Barreiro-Iglesias, R.; Concheiro, A. Imprinted soft contact lenses as norfloxacin delivery systems. J. Control. Release 2006, 113, 236–244. [Google Scholar] [CrossRef]
- Ciardelli, G.; Cioni, B.; Cristallini, C.; Barbani, N.; Silvestri, D.; Giusti, P. Acrylic polymeric nanospheres for the release and recognition ofmolecules of clinical interest. Biosens. Bioelectron. 2004, 20, 1083–1090. [Google Scholar] [CrossRef]
- Esfandyari-Manesh, M.; Javanbakht, M.; Atyabi, F.; Mohammadi, A.; Mohammadi, S.; Akbari-Adergani, B.; Dinarvand, R. Dipyridamole recognition and controlled release by uniformly sized molecularly imprinted nanospheres. Mater. Sci. Eng. C 2011, 31, 1692–1699. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mousavi, Y.; Hockerstedt, K. Diet and breast cancer. Acta Oncol. 1992, 31, 175–181. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; Takats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef]
- Zheng, Y.; Haworth, I.S.; Zuo, Z.; Chow, M.S.S.; Chow, A.H.L. Physicochemical and structural characterization of quer-cetin-b-cyclodextrin complexes. J. Pharm. Sci. 2005, 94, 1079–1089. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B 2010, 80, 184–192. [Google Scholar] [CrossRef]
- Lee, D.H.; Sim, G.S.; Kim, J.H.; Lee, G.S.; Pyo, H.B.; Lee, B.C. Preparation and characterization of quercetin-loadedpolymethyl methacrylate microcapsules usinga polyol-in-oil-in-polyol emulsion solventevaporation method. J. Pharm. Pharmacol. 2007, 59, 1611–1620. [Google Scholar]
- Barreto, A.C.H.; Santiago, V.R.; Mazzetto, S.E.; Denardin, J.C.; Lavìn, R.; Mele, G.; Ribeiro, M.E.N.P.; Vieira, I.G.P.; Gonalves, T.; Ricardo, N.M.P.S.; Fechine, P.B.A. Magnetic nanoparticles for a new drug delivery systemto control quercetin releasing for cancer chemotherapy. J. Nanopart. Res. 2011, 13, 6545–6553. [Google Scholar]
- Flavin, K.; Resmini, M. Imprinted nanomaterials: A new class of synthetic receptors. Anal. Bioanal. Chem. 2009, 393, 437–444. [Google Scholar] [CrossRef]
- Spégel, P.; Schweitz, L.; Nilsson, S. Selectivity toward multiple predetermined targets in nanoparticle capillary electrochromatography. Anal. Chem. 2003, 75, 6608–6613. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Molecularly imprinted polymers for drug delivery. J. Chromatogr. B 2004, 804, 231–245. [Google Scholar] [CrossRef]
- Ye, L.; Mosbach, K. Molecular imprinting: Synthetic materials as substitutes for biological antibodies and receptors. Chem. Mater. 2008, 20, 859–868. [Google Scholar] [CrossRef]
- Boos, K.S.; Fleischer, C.T. Multidimensional on-line solid-phase extraction (SPE) using restricted access materials (RAM) in combination with molecular imprinted polymers (MIP). J. Anal. Chem. 2001, 371, 16–20. [Google Scholar]
- Gore, M.A.; Karmalkar, R.N.; Kulkarni, M.G. Enhanced capacities and selectivities for cholesterol in aqueous media by molecular imprinting: Role of novel crosslinkers. J. Chromatogr. B 2004, 804, 211–221. [Google Scholar] [CrossRef]
- Pitarresi, G.; Pierro, P.; Giammona, G.; Iemma, F.; Muzzalupo, R.; Picci, N. Drug release from α,β-poly(N-2-hydroxyethyl)-dl-aspartamide-based microparticles. Biomaterials 2004, 25, 4333–4343. [Google Scholar] [CrossRef]
- Puoci, F.; Cirillo, G.; Curcio, M.; Iemma, F.; Parisi, O.I.; Castiglione, M.; Picci, N. Molecularly imprinted polymers for α-tocopherol delivery. Drug Deliv. 2008, 15, 253–258. [Google Scholar] [CrossRef]
- Doak, S.H.; Griffiths, S.M.; Manshian, B.; Singh, N.; Williams, P.M.; Brown, A.P.; Jenkins, G.J.S. Confounding experimental considerations in nanogenotoxicology. Mutagenesis 2009, 24, 285–293. [Google Scholar] [CrossRef]
- Liu, Y.; Nair, M.G. An efficient and economical MTT assay for determining the antioxidant activity of plant natural product extracts and pure compounds. J. Nat. Prod. 2010, 73, 1193–1195. [Google Scholar] [CrossRef]
- Priyadarsini, R.V.; Murugan, R.S.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2012, 649, 84–91. [Google Scholar]
- Jagtap, S.; Meganathan, K.; Wagh, V.; Winkler, J.; Hescheler, J.; Sachinidis, A. Chemoprotective mechanism of the natural compounds, epigallocatechin-3-O-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr. Med. Chem. 2009, 16, 1451–1462. [Google Scholar] [CrossRef]
- AlaaEddeen, M.; Seufi, A.M.; Ibrahim, S.S.; Elmaghraby, T.K.; Hafez, E.E. Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/antioxidant activity: Molecular and histological evidences. J. Exp. Clin. Cancer Res. 2009, 28. [Google Scholar] [CrossRef]
- Boly, R.; Gras, T.; Lamkami, T.; Guissou, P.; Serteyn, D.; Kiss, R.; Dubois, J. Quercetin inhibits a large panel of kinases implicated in cancer cell biology. Int. J. Oncol. 2011, 38, 833–842. [Google Scholar]
- Estella-Hermoso de Mendoza, A.; Préat, V.; Mollinedo, F.; Blanco-Prieto, M.J. In vitro and in vivo efficacy of edelfosine-loaded lipid nanoparticles against glioma. J. Control. Release 2011, 156, 421–426. [Google Scholar] [CrossRef]
- Song, X.; Li, J.; Wang, J.; Chen, L. Quercetin molecularly imprinted polymers: Preparation, recognition characteristics and properties as sorbent for solid phase extraction. Talanta 2009, 80, 694–702. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Curcio, M.; Cirillo, G.; Parisi, O.I.; Iemma, F.; Picci, N.; Puoci, F. Quercetin-Imprinted Nanospheres as Novel Drug Delivery Devices. J. Funct. Biomater. 2012, 3, 269-282. https://doi.org/10.3390/jfb3020269
Curcio M, Cirillo G, Parisi OI, Iemma F, Picci N, Puoci F. Quercetin-Imprinted Nanospheres as Novel Drug Delivery Devices. Journal of Functional Biomaterials. 2012; 3(2):269-282. https://doi.org/10.3390/jfb3020269
Chicago/Turabian StyleCurcio, Manuela, Giuseppe Cirillo, Ortensia Ilaria Parisi, Francesca Iemma, Nevio Picci, and Francesco Puoci. 2012. "Quercetin-Imprinted Nanospheres as Novel Drug Delivery Devices" Journal of Functional Biomaterials 3, no. 2: 269-282. https://doi.org/10.3390/jfb3020269
APA StyleCurcio, M., Cirillo, G., Parisi, O. I., Iemma, F., Picci, N., & Puoci, F. (2012). Quercetin-Imprinted Nanospheres as Novel Drug Delivery Devices. Journal of Functional Biomaterials, 3(2), 269-282. https://doi.org/10.3390/jfb3020269









